Abstract
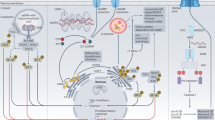
The recognition of pathogenic DNA is important to the initiation of antiviral responses. Here we report the identification of DDX41, a member of the DEXDc family of helicases, as an intracellular DNA sensor in myeloid dendritic cells (mDCs). Knockdown of DDX41 expression by short hairpin RNA blocked the ability of mDCs to mount type I interferon and cytokine responses to DNA and DNA viruses. Overexpression of both DDX41 and the membrane-associated adaptor STING together had a synergistic effect in promoting Ifnb promoter activity. DDX41 bound both DNA and STING and localized together with STING in the cytosol. Knockdown of DDX41 expression blocked activation of the mitogen-activated protein kinase TBK1 and the transcription factors NF-κB and IRF3 by B-form DNA. Our results suggest that DDX41 is an additional DNA sensor that depends on STING to sense pathogenic DNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
17 November 2011
In the version of this article initially published, the sensor DDX41 was suggested to recognize Z-form DNA in addition to conventional B-form DNA. Although GC-rich DNA has the potential to adopt a left-handed Z-DNA conformation under conditions of high salt or ethanol, whether the GC-rich oligonucleotides used the original study actually adopted the Z-DNA conformation remains uncertain. Therefore, the article has been corrected throughout to reflect whether poly(dA:dT) or poly(dG:dC) was used to stimulate cells. The errors have been corrected in the HTML and PDF versions of the article.
References
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Takeuchi, O. & Akira, S. Recognition of viruses by innate immunity. Immunol. Rev. 220, 214–224 (2007).
Blasius, A.L. & Beutler, B. Intracellular toll-like receptors. Immunity 32, 305–315 (2010).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Myong, S. et al. Cytosolic viral sensor RIG-I is a 5′-triphosphate-dependent translocase on double-stranded RNA. Science 323, 1070–1074 (2009).
Pippig, D.A. et al. The regulatory domain of the RIG-I family ATPase LGP2 senses double-stranded RNA. Nucleic Acids Res. 37, 2014–2025 (2009).
Takeuchi, O. & Akira, S. Innate immunity to virus infection. Immunol. Rev. 227, 75–86 (2009).
Chiu, Y.H., Macmillan, J.B. & Chen, Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10, 1065–1072 (2009).
Roberts, T.L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Fernandes-Alnemri, T., Datta, J.W., Wu, P.J. & Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Bürckstümmer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Rathinam, V.A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010).
Jones, J.W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 107, 9771–9776 (2010).
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008).
Ishikawa, H. & Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Ishikawa, H., Ma, Z. & Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 (2010).
Kim, T. et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 107, 15181–15186 (2010).
Zhang, Z. et al. DDX1, DDX21 and DHX36 Helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity 34, 866–878 (2011).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007).
Ishii, K.J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008).
Wang, Z. et al. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl. Acad. Sci. USA 105, 5477–5482 (2008).
Sun, Q. et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24, 633–642 (2006).
Kumar, H. et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203, 1795–1803 (2006).
Cheng, G., Zhong, J., Chung, J. & Chisari, F.V. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc. Natl. Acad. Sci. USA 104, 9035–9040 (2007).
Acknowledgements
We thank M. Wentz and S. Watowich for critical reading; T. Zal for technical support with confocal microscopy; G. Cheng and K. Parvatiyar for suggestions on the experiments; and all colleagues in our laboratory.
Author information
Authors and Affiliations
Contributions
Z.Z. designed and did most of experiments; B.Y., M.B., N.L. and T.K. helped with experiments; and Y.-J.L. designed the research and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Table 1 and Methods (PDF 470 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Yuan, B., Bao, M. et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol 12, 959–965 (2011). https://doi.org/10.1038/ni.2091
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.2091
This article is cited by
-
MicroRNA-218-5p-Ddx41 axis restrains microglia-mediated neuroinflammation through downregulating type I interferon response in a mouse model of Parkinson’s disease
Journal of Translational Medicine (2024)
-
LNCGM1082-mediated NLRC4 activation drives resistance to bacterial infection
Cellular & Molecular Immunology (2023)
-
Ribosome profiling analysis reveals the roles of DDX41 in translational regulation
International Journal of Hematology (2023)
-
TRIM18 is a critical regulator of viral myocarditis and organ inflammation
Journal of Biomedical Science (2022)
-
A novel mechanism for macrophage pyroptosis in rheumatoid arthritis induced by Pol β deficiency
Cell Death & Disease (2022)