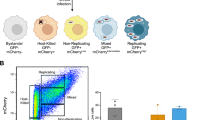
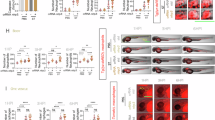
Abstract
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a virulent pathogen that induces rapid host death. Here we observed that host survival after infection with S. Typhimurium was enhanced in the absence of type I interferon signaling, with improved survival of mice deficient in the receptor for type I interferons (Ifnar1−/− mice) that was attributed to macrophages. Although there was no impairment in cytokine expression or inflammasome activation in Ifnar1−/− macrophages, they were highly resistant to S. Typhimurium–induced cell death. Specific inhibition of the kinase RIP1 or knockdown of the gene encoding the kinase RIP3 prevented the death of wild-type macrophages, which indicated that necroptosis was a mechanism of cell death. Finally, RIP3-deficient macrophages, which cannot undergo necroptosis, had similarly less death and enhanced control of S. Typhimurium in vivo. Thus, we propose that S. Typhimurium induces the production of type I interferon, which drives necroptosis of macrophages and allows them to evade the immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jones, B.D. & Falkow, S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14, 533–561 (1996).
Vidal, S. et al. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182, 655–666 (1995).
Sad, S. et al. Pathogen proliferation governs the magnitude but compromises the function of CD8 T cells. J. Immunol. 180, 5853–5861 (2008).
Luu, R.A. et al. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J. Immunol. 177, 1516–1525 (2006).
Albaghdadi, H., Robinson, N., Finlay, B., Krishnan, L. & Sad, S. Selectively reduced intracellular proliferation of Salmonella enterica serovar typhimurium within APCs limits antigen presentation and development of a rapid CD8 T cell response. J. Immunol. 183, 3778–3787 (2009).
Vidric, M., Bladt, A.T., Dianzani, U. & Watts, T.H. Role for inducible costimulator in control of Salmonella enterica serovar Typhimurium infection in mice. Infect. Immun. 74, 1050–1061 (2006).
O'Brien, A.D., Scher, I. & Formal, S.B. Effect of silica on the innate resistance of inbred mice to Salmonella typhimurium infection. Infect. Immun. 25, 513–520 (1979).
Salcedo, S.P., Noursadeghi, M., Cohen, J. & Holden, D.W. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell. Microbiol. 3, 587–597 (2001).
Lindgren, S.W., Stojiljkovic, I. & Heffron, F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93, 4197–4201 (1996).
Stockinger, S. & Decker, T. Novel functions of type I interferons revealed by infection studies with Listeria monocytogenes. Immunobiology 213, 889–897 (2008).
Hersh, D. et al. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96, 2396–2401 (1999).
Brennan, M.A. & Cookson, B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40 (2000).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119 (2005).
Hitomi, J. et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135, 1311–1323 (2008).
Cho, Y.S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Scharton, K.T., Afonso, L.C., Wysocka, M., Trinchieri, G. & Scott, P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154, 5320–5330 (1995).
Gross, O., Thomas, C.J., Guarda, G. & Tschopp, J. The inflammasome: an integrated view. Immunol. Rev. 243, 136–151 (2011).
Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Biron, C.A. Interferons α and β as immune regulators–a new look. Immunity 14, 661–664 (2001).
Mancuso, G. et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J. Immunol. 178, 3126–3133 (2007).
Auerbuch, V., Brockstedt, D.G., Meyer-Morse, N., O'Riordan, M. & Portnoy, D.A. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200, 527–533 (2004).
O'Connell, R.M. et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200, 437–445 (2004).
Thyrell, L. et al. Interferon α-induced apoptosis in tumor cells is mediated through the phosphoinositide 3-kinase/mammalian target of rapamycin signaling pathway. J. Biol. Chem. 279, 24152–24162 (2004).
Miao, E.A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Gobeil, S., Boucher, C.C., Nadeau, D. & Poirier, G.G. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ. 8, 588–594 (2001).
Fink, S.L. & Cookson, B.T. Pyroptosis and host cell death responses during Salmonella infection. Cell. Microbiol. 9, 2562–2570 (2007).
Galluzzi, L. & Kroemer, G. Necroptosis: a specialized pathway of programmed necrosis. Cell 135, 1161–1163 (2008).
Degterev, A. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321 (2008).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Vanlangenakker, N. et al. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 18, 656–665 (2011).
Lee, T.H., Shank, J., Cusson, N. & Kelliher, M.A. The kinase activity of Rip1 is not required for tumor necrosis factor-α-induced IκB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J. Biol. Chem. 279, 33185–33191 (2004).
Vandenabeele, P., Declercq, W., Van Herreweghe, F. & Vanden Berghe, T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci. Signal. 3, re4 (2010).
McComb, S. et al. cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ. advance online publication, doi:10.1038/cdd.2012.59 (11 May 2012).
van Faassen, H., Dudani, R., Krishnan, L. & Sad, S. Prolonged antigen presentation, APC−, and CD8+ T cell turnover during mycobacterial infection: comparison with Listeria monocytogenes. J. Immunol. 172, 3491–3500 (2004).
Vince, J.E. et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36, 215–227 (2012).
Labbé, K., McIntire, C.R., Doiron, K., Leblanc, P.M. & Saleh, M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity 35, 897–907 (2011).
Knodler, L.A. & Finlay, B.B. Salmonella and apoptosis: to live or let die? Microbes Infect. 3, 1321–1326 (2001).
Monack, D.M. et al. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192, 249–258 (2000).
Lara-Tejero, M. et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203, 1407–1412 (2006).
Raupach, B., Peuschel, S.K., Monack, D.M. & Zychlinsky, A. Caspase-1-mediated activation of interleukin-1β (IL-1β) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect. Immun. 74, 4922–4926 (2006).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
He, S., Liang, Y., Shao, F. & Wang, X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc. Natl. Acad. Sci. USA 108, 20054–20059 (2011).
Upton, J.W., Kaiser, W.J. & Mocarski, E.S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010).
Mack, C., Sickmann, A., Lembo, D. & Brune, W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl. Acad. Sci. USA 105, 3094–3099 (2008).
Sing, A. et al. Bacterial induction of β interferon in mice is a function of the lipopolysaccharide component. Infect. Immun. 68, 1600–1607 (2000).
Pejcic-Karapetrovic, B. et al. Pregnancy impairs the innate immune resistance to Salmonella typhimurium leading to rapid fatal infection. J. Immunol. 179, 6088–6096 (2007).
Wong, C.E., Sad, S. & Coombes, B.K. Salmonella enterica serovar typhimurium exploits Toll-like receptor signaling during the host-pathogen interaction. Infect. Immun. 77, 4750–4760 (2009).
Khan, R. et al. Refinement of the genetics of the host response to Salmonella infection in MOLF/Ei: regulation of type 1 IFN and TRP3 pathways by Ity2. Genes Immun. 13, 175–183 (2012).
Gordon, M.A. et al. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Clin. Infect. Dis. 50, 953–962 (2010).
Acknowledgements
We thank K. Murali-Krishna (Emory University) for Ifnar−/− mice; V. Dixit (Genentech) for Rip3−/− mice; B. Beutler (The University of Texas Southwestern Medical Center) for the L929-ISRE cell line; and S. Thurston for technical help. Supported by the Canadian Institutes of Health Research (S.S.), the Ontario Institute of Cancer Research (L.K.) and the National Research Council of Canada.
Author information
Authors and Affiliations
Contributions
N.R., S.M., R.M. and R.D. performed experiments and analyzed the data. N.R., S.M., L.K. and S.S. designed the experiments and wrote the manuscript. S.S. and L.K. conceptualized the project, obtained research funding and provided reagents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 205 kb)
Rights and permissions
About this article
Cite this article
Robinson, N., McComb, S., Mulligan, R. et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol 13, 954–962 (2012). https://doi.org/10.1038/ni.2397
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.2397
This article is cited by
-
A novel proteomic-based model for predicting colorectal cancer with Schistosoma japonicum co‐infection by integrated bioinformatics analysis and machine learning
BMC Medical Genomics (2023)
-
Fusobacterium nucleatum triggers proinflammatory cell death via Z-DNA binding protein 1 in apical periodontitis
Cell Communication and Signaling (2022)
-
The Lck inhibitor, AMG-47a, blocks necroptosis and implicates RIPK1 in signalling downstream of MLKL
Cell Death & Disease (2022)
-
Identification of perturbed pathways rendering susceptibility to tuberculosis in type 2 diabetes mellitus patients using BioNSi simulation of integrated networks of implicated human genes
Journal of Biosciences (2022)
-
Type I interferon signaling and macrophages: a double-edged sword?
Cellular & Molecular Immunology (2021)