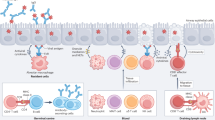
Abstract
Respiratory viruses are frequent causes of repeated common colds, bronchitis and pneumonia, which often occur unpredictably as epidemics and pandemics. Despite those decimating effects on health and decades of intensive research, treatments remain largely supportive. The only commonly available vaccines are against influenza virus, and even these need improvement. The lung shares some features with other mucosal sites, but preservation of its especially delicate anatomical structures necessitates a fine balance of pro- and anti-inflammatory responses; well-timed, appropriately placed and tightly regulated T cell and B cell responses are essential for protection from infection and limitation of symptoms, whereas poorly regulated inflammation contributes to tissue damage and disease. Recent advances in understanding adaptive immunity should facilitate vaccine development and reduce the global effect of respiratory viruses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Kim Caesar/Nature Publishing Group

Kim Caesar/Nature Publishing Group

Kim Caesar/Nature Publishing Group
Similar content being viewed by others
References
Heikkinen, T. & Järvinen, A. The common cold. Lancet 361, 51–59 (2003).
Esposito, S. et al. Clinical and socio-economic impact of influenza and respiratory syncytial virus infection on healthy children and their households. Clin. Microbiol. Infect. 11, 933–936 (2005).
Nair, H. et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375, 1545–1555 (2010).
Lee, M.-S., Walker, R.E. & Mendelman, P.M. Medical burden of respiratory syncytial virus and parainfluenza virus type 3 infection among US children. Implications for design of vaccine trials. Hum. Vaccin. 1, 6–11 (2005).
Ruuskanen, O., Lahti, E., Jennings, L.C. & Murdoch, D.R. Viral pneumonia. Lancet 377, 1264–1275 (2011).
Asner, S.A. et al. Clinical severity of rhinovirus/enterovirus compared to other respiratory viruses in children. Influenza Other Respir. Viruses 8, 436–442 (2014).
Greenberg, S. Update on rhinovirus and coronavirus infections. Semin. Respir. Crit. Care Med. 32, 433–446 (2011).
Kroll, J. & Weinberg, A. Human metapneumovirus. Semin. Respir. Crit. Care Med. 32, 447–453 (2011).
Bautista, E. et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N. Engl. J. Med. 362, 1708–1719 (2010).
Peiris, J.S.M., Yuen, K.Y., Osterhaus, A.D.M.E. & Stöhr, K. The severe acute respiratory syndrome. N. Engl. J. Med. 349, 2431–2441 (2003).
Guery, B. et al. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet 381, 2265–2272 (2013).
Manzoli, L., Ioannidis, J.P.A., Flacco, M.E., Vito, C.D. & Villari, P. Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses. Hum. Vaccin. Immunother. 8, 851–862 (2012).
Plotkin, S.A. Complex correlates of protection after vaccination. Clin. Infect. Dis. 56, 1458–1465 (2013).
Crowe, J.E. Jr. & Williams, J.V. Immunology of viral respiratory tract infection in infancy. Paediatr. Respir. Rev. 4, 112–119 (2003).
Goronzy, J.J. & Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428–436 (2013).
Couch, R.B. & Kasel, J.A. Immunity to influenza in man. Annu. Rev. Microbiol. 37, 529–549 (1983).
Glanville, N. et al. Cross-serotype immunity induced by immunization with a conserved rhinovirus capsid protein. PLoS Pathog. 9, e1003669 (2013).
Openshaw, P.J. & Chiu, C. Protective and dysregulated T cell immunity in RSV infection. Curr. Opin. Virol. 3, 468–474 (2013).
Woodland, D.L. & Randall, T.D. Anatomical features of anti-viral immunity in the respiratory tract. Semin. Immunol. 16, 163–170 (2004).
Raz, E. Organ-specific regulation of innate immunity. Nat. Immunol. 8, 3–4 (2007).
Snelgrove, R.J. et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 9, 1074–1083 (2008).
Li, Y., Dinwiddie, D.L., Harrod, K.S., Jiang, Y. & Kim, K.C. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L558–L563 (2010).
Monticelli, L.A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 12, 1045–1054 (2011).
Hansel, T.T., Johnston, S.L. & Openshaw, P.J. Microbes and mucosal immune responses in asthma. Lancet 381, 861–873 (2013).
Brown, E.M., Sadarangani, M. & Finlay, B.B. The role of the immune system in governing host-microbe interactions in the intestine. Nat. Immunol. 14, 660–667 (2013).
McDermott, A.J. & Huffnagle, G.B. The microbiome and regulation of mucosal immunity. Immunology 142, 24–31 (2014).
Han, M.K. et al. Significance of the microbiome in obstructive lung disease. Thorax 67, 456–463 (2012).
Wang, J. et al. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat. Commun. 4, 2106 (2013).
Cleaver, J.O. et al. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal Immunol. 7, 78–88 (2014).
Barlow, P.G., Findlay, E.G., Currie, S.M. & Davidson, D.J. Antiviral potential of cathelicidins. Future Microbiol. 9, 55–73 (2013).
Vareille, M., Kieninger, E., Edwards, M.R. & Regamey, N. The airway epithelium: soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 24, 210–229 (2011).
Yoo, J.-K., Kim, T.S., Hufford, M.M. & Braciale, T.J. Viral infection of the lung: host response and sequelae. J. Allergy Clin. Immunol. 132, 1263–1276 (2013).
Guilliams, M., Lambrecht, B.N. & Hammad, H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 6, 464–473 (2013).
Legge, K.L. & Braciale, T.J. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18, 265–277 (2003).
Legge, K.L. & Braciale, T.J. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity 23, 649–659 (2005).
Culley, F.J. Natural killer cells in infection and inflammation of the lung. Immunology 128, 151–163 (2009).
Kohlmeier, J.E. et al. Interferons regulate cytolytic activity of memory CD8+ T cells in the lung airways during respiratory virus challenge. Immunity 33, 96–105 (2010).
González-Navajas, J.M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Plotkin, S.A. & Plotkin, S.A. Correlates of vaccine-induced immunity. Clin. Infect. Dis. 47, 401–409 (2008).
Morokutti, A. et al. Validation of the modified hemagglutination inhibition assay (mHAI), a robust and sensitive serological test for analysis of influenza virus-specific immune response. J. Clin. Virol. 56, 323–330 (2013).
Grund, S., Adams, O., Wählisch, S. & Schweiger, B. Comparison of hemagglutination inhibition assay, an ELISA-based micro-neutralization assay and colorimetric microneutralization assay to detect antibody responses to vaccination against influenza A H1N1 2009 virus. J. Virol. Methods 171, 369–373 (2011).
Van Remmerden, Y. et al. An improved respiratory syncytial virus neutralization assay based on the detection of green fluorescent protein expression and automated plaque counting. Virol. J. 9, 253 (2012).
Falsey, A.R., Formica, M.A. & Walsh, E.E. Microneutralization assay for the measurement of neutralizing antibodies to human metapneumovirus. J. Clin. Virol. 46, 314–317 (2009).
Jegaskanda, S., Reading, P.C. & Kent, S.J. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J. Immunol. 193, 469–475 (2014).
Gupta, N. et al. Affinity-purified respiratory syncytial virus antibodies from intravenous immunoglobulin exert potent antibody-dependent cellular cytotoxicity. PLoS ONE 8, e69390 (2013).
Renegar, K.B., Small, P.A., Boykins, L.G. & Wright, P.F. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol. 173, 1978–1986 (2004).
Van Riet, E., Ainai, A., Suzuki, T. & Hasegawa, H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine 30, 5893–5900 (2012).
Asahi, Y. et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J. Immunol. 168, 2930–2938 (2002).
Asahi-Ozaki, Y. et al. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J. Med. Virol. 74, 328–335 (2004).
Spiekermann, G.M. et al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life functional expression of FcRn in the mammalian lung. J. Exp. Med. 196, 303–310 (2002).
The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102, 531–537 (1998).
Batista, F.D. & Harwood, N.E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 9, 15–27 (2009).
Moyron-Quiroz, J.E. et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 25, 643–654 (2006).
MacLennan, I.C.M. & Vinuesa, C.G. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity 17, 235–238 (2002).
McNamara, P.S. et al. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax 68, 76–81 (2013).
Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 8, 421–434 (2008).
McHeyzer-Williams, M., Okitsu, S., Wang, N. & McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 12, 24–34 (2012).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Nutt, S.L. & Tarlinton, D.M. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat. Immunol. 12, 472–477 (2011).
Boyden, A.W., Legge, K.L. & Waldschmidt, T.J. Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses. PLoS ONE 7, e40733 (2012).
Chu, V.T. & Berek, C. The establishment of the plasma cell survival niche in the bone marrow. Immunol. Rev. 251, 177–188 (2013).
Liang, B., Hyland, L. & Hou, S. Nasal-associated lymphoid tissue Is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J. Virol. 75, 5416–5420 (2001).
Rookhuizen, D.C. & DeFranco, A.L. Toll-like receptor 9 signaling acts on multiple elements of the germinal center to enhance antibody responses. Proc. Natl. Acad. Sci. USA 111, E3224–E3233 (2014).
Chevrier, S. et al. The BTB-ZF transcription factor Zbtb20 is driven by Irf4 to promote plasma cell differentiation and longevity. J. Exp. Med. 211, 827–840 (2014).
Goodnow, C.C., Vinuesa, C.G., Randall, K.L., Mackay, F. & Brink, R. Control systems and decision making for antibody production. Nat. Immunol. 11, 681–688 (2010).
Zuccarino-Catania, G.V. et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 15, 631–637 (2014).
Yewdell, J.W. Viva la Revolución: rethinking influenza A virus antigenic drift. Curr. Opin. Virol. 1, 177–183 (2011).
McIntyre, C.L., Knowles, N.J. & Simmonds, P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J. Gen. Virol. 94, 1791–1806 (2013).
Martinelli, M. et al. Phylogeny and population dynamics of respiratory syncytial virus (Rsv) A and B. Virus Res. 189, 293–302 (2014).
Hall, C., Walsh, E., Long, C. & Schnabel, K. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163, 693–698 (1991).
Munir, S. et al. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog. 7, e1001336 (2011).
Chirkova, T. et al. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J. Virol. 87, 13466–13479 (2013).
Falsey, A.R., Singh, H.K. & Walsh, E.E. Serum antibody decay in adults following natural respiratory syncytial virus infection. J. Med. Virol. 78, 1493–1497 (2006).
Pratama, A. & Vinuesa, C.G. Control of TFH cell numbers: why and how? Immunol. Cell Biol. 92, 40–48 (2014).
Lee, S.K. et al. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J. Exp. Med. 208, 1377–1388 (2011).
Nurieva, R.I. et al. Bcl6 mediates the development of T follicular helper cells. Science 325, 1001–1005 (2009).
Cannons, J.L., Lu, K.T. & Schwartzberg, P.L. T follicular helper cell diversity and plasticity. Trends Immunol. 34, 200–207 (2013).
Choi, Y.S. et al. Bcl6 dependent T follicular helper cell differentiation diverges from effector cell differentiation during priming and depends on the gene Icos. Immunity 34, 932–946 (2011).
Tubo, N.J. et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell 153, 785–796 (2013).
Ballesteros-Tato, A. et al. Interleukin-2 Inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 36, 847–856 (2012).
Morita, R. et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121 (2011).
Bentebibel, S.-E. et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 5, 176ra32–176ra32 (2013).
Spensieri, F. et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc. Natl. Acad. Sci. USA 110, 14330–14335 (2013).
Baumjohann, D. et al. The microRNA cluster miR-17∼92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nat. Immunol. 14, 840–848 (2013).
Kang, S.G. et al. MicroRNAs of the miR-17∼92 family are critical regulators of TFH differentiation. Nat. Immunol. 14, 849–857 (2013).
Purwar, R. et al. Resident memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE 6, e16245 (2011).
Zhao, Y. et al. High levels of virus-specific CD4+ T cells predict severe pandemic influenza A virus infection. Am. J. Respir. Crit. Care Med. 186, 1292–1297 (2012).
Heidema, J. et al. Dynamics of human respiratory virus-specific CD8+ T cell responses in blood and airways during episodes of common cold. J. Immunol. 181, 5551–5559 (2008).
Christensen, J.P., Doherty, P.C., Branum, K.C. & Riberdy, J.M. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8+ T-cell memory. J. Virol. 74, 11690–11696 (2000).
Graham, B.S., Bunton, L.A., Wright, P.F. & Karzon, D.T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88, 1026–1033 (1991).
Fishaut, M., Tubergen, D. & McIntosh, K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J. Pediatr. 96, 179–186 (1980).
Wilkinson, T.M. et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18, 274–280 (2012).
Sridhar, S. et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 19, 1305–1312 (2013).
Chirkova, T.V. et al. Memory T-cell immune response in healthy young adults vaccinated with live attenuated influenza A (H5N2) vaccine. Clin. Vaccine Immunol. 18, 1710–1718 (2011).
Channappanavar, R., Fett, C., Zhao, J., Meyerholz, D. & Perlman, S. Virus-specific memory CD8 T cells provide substantial protection from lethal SARS-CoV infection. J. Virol. 88, 11034–11044 (2014).
Herd, K.A., Nelson, M., Mahalingam, S. & Tindle, R.W. Pulmonary infection of mice with human metapneumovirus induces local cytotoxic T-cell and immunoregulatory cytokine responses similar to those seen with human respiratory syncytial virus. J. Gen. Virol. 91, 1302–1310 (2010).
Braciale, T.J., Sun, J. & Kim, T.S. Regulating the adaptive immune response to respiratory virus infection. Nat. Rev. Immunol. 12, 295–305 (2012).
Krishnamoorthy, N. et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat. Med. 18, 1525–1530 (2012).
Bystrom, J., Al-Adhoubi, N., Al-Bogami, M., Jawad, A. & Mageed, R. Th17 lymphocytes in respiratory syncytial virus infection. Viruses 5, 777–791 (2013).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Cauley, L.S. & Lefrançois, L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol. 6, 14–23 (2013).
Schenkel, J.M. et al. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346, 98–101 (2014).
Schenkel, J.M., Fraser, K.A., Vezys, V. & Masopust, D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 14, 509–513 (2013).
Teijaro, J.R. et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 (2011).
Mackay, L.K. et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 (2013).
Ray, S.J. et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20, 167–179 (2004).
Kim, T.S., Gorski, S.A., Hahn, S., Murphy, K.M. & Braciale, T.J. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8+ T cell differentiation by a CD24-dependent mechanism. Immunity 40, 400–413 (2014).
Slütter, B., Pewe, L.L., Kaech, S.M. & Harty, J.T. Lung airway-surveilling CXCR3hi memory CD8+ T cells are critical for protection against influenza A virus. Immunity 39, 939–948 (2013).
Mikhak, Z., Strassner, J.P. & Luster, A.D. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J. Exp. Med. 210, 1855–1869 (2013).
Iijima, N. & Iwasaki, A. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346, 93–98 (2014).
Joshi, N.S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Li, M.O. & Flavell, R.A. TGF-β: a master of all T cell trades. Cell 134, 392–404 (2008).
Carlson, C.M. et al. Transforming growth factor-β: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog. 6, e1001136 (2010).
Wu, T. et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 95, 215–224 (2013).
Everitt, A.R. et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 484, 519–523 (2012).
Wakim, L.M., Gupta, N., Mintern, J.D. & Villadangos, J.A. Enhanced survival of lung tissue-resident memory CD8+ T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 14, 238–245 (2013).
Bruder, D., Srikiatkhachorn, A. & Enelow, R.I. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 19, 147–155 (2006).
De Jong, M.D. et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207 (2006).
Walsh, K.B. et al. Animal model of respiratory syncytial virus: CD8+ T cells cause cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J. Virol. 88, 6281–6293 (2014).
Cannon, M.J., Openshaw, P.J. & Askonas, B.A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168, 1163–1168 (1988).
Smyth, R.L. & Openshaw, P.J. Bronchiolitis. Lancet 368, 312–322 (2006).
Tscherne, D.M. & García-Sastre, A. Virulence determinants of pandemic influenza viruses. J. Clin. Invest. 121, 6–13 (2011).
Barik, S. Respiratory syncytial virus mechanisms to interfere with type 1 interferons. Curr. Top. Microbiol. Immunol. 372, 173–191 (2013).
Tripp, R.A., Jones, L.P., Zheng, H., Murphy, P.M. & Anderson, L.J. CX3C chemokine mimcry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2, 732–738 (2001).
Welliver, T.P. et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195, 1126–1136 (2007).
Moghaddam, A. et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat. Med. 12, 905–907 (2006).
Delgado, M.F. et al. Lack of antibody affinity maturation due to poor Toll stimulation led to enhanced RSV disease. Nat. Med. 15, 34–41 (2009).
Polack, F.P. et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 196, 859–865 (2002).
de Swart, R.L. et al. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J. Virol. 76, 11561–11569 (2002).
Loebbermann, J., Durant, L., Thornton, H., Johansson, C. & Openshaw, P.J. Defective immunoregulation in RSV vaccine-augmented viral lung disease restored by selective chemoattraction of regulatory T cells. Proc. Natl. Acad. Sci. USA 110, 2987–2992 (2013).
Mukherjee, S. et al. IL-17–induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am. J. Pathol. 179, 248–258 (2011).
Stoppelenburg, A.J. et al. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS ONE 8, e78461 (2013).
Sun, J., Madan, R., Karp, C.L. & Braciale, T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 15, 277–284 (2009).
León, B., Bradley, J.E., Lund, F.E., Randall, T.D. & Ballesteros-Tato, A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat. Commun. 5, 3495 (2014).
Loebbermann, J. et al. Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol. 5, 161–172 (2012).
Tricco, A.C. et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 11, 153 (2013).
Belshe, R.B. et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 181, 1133–1137 (2000).
Schmidt, A. Progress in respiratory virus vaccine development. Semin. Respir. Crit. Care Med. 32, 527–540 (2011).
Chiu, C. et al. Cross-reactive humoral responses to influenza and their implications for a universal vaccine. Ann. NY Acad. Sci. 1283, 13–21 (2013).
McLellan, J.S. et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342, 592–598 (2013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chiu, C., Openshaw, P. Antiviral B cell and T cell immunity in the lungs. Nat Immunol 16, 18–26 (2015). https://doi.org/10.1038/ni.3056
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.3056
This article is cited by
-
Role of T Lymphocyte Activation Profile in Predicting SARS-CoV-2 Severity: Experience from Tertiary Care Centre of North India
Indian Journal of Hematology and Blood Transfusion (2023)
-
Teleost swim bladder, an ancient air-filled organ that elicits mucosal immune responses
Cell Discovery (2022)
-
Type II alveolar cell MHCII improves respiratory viral disease outcomes while exhibiting limited antigen presentation
Nature Communications (2021)
-
Viral infections and wheezing–asthma inception in childhood: is there a role for immunomodulation by oral bacterial lysates?
Clinical and Translational Allergy (2020)
-
Intranasal immunization with outer membrane vesicle pertussis vaccine confers broad protection through mucosal IgA and Th17 responses
Scientific Reports (2020)