Abstract
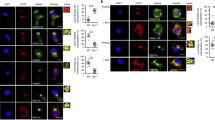
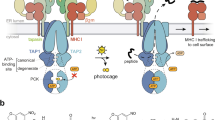
In dendritic cells (DCs), peptides derived from internalized particulate substrates are efficiently cross-presented by major histocompatibility complex (MHC) class I molecules. Exogenous soluble antigens are also presented by DCs but with substantially lower efficiency. Here we show that particulate and soluble antigens use different transport pathways. Particulate antigens have been shown to access peripheral endoplasmic reticulum (ER)–like phagosomes that are competent for cross-presentation, whereas we show here that soluble proteins that escape proteolysis enter the lumen of the ER. From there, they may be translocated into the cytosol by the pathway established for ER-associated degradation and their derived peptides may be transported back into the ER for binding by MHC class I molecules. MHC class I presentation involving the constitutive retrograde transport of soluble proteins to the ER by DCs may facilitate DC tolerance to components of their extracellular environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mueller, S.N., Jones, C.M., Smith, C.M., Heath, W.R. & Carbone, F.R. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 195, 651–656 (2002).
Gredmark, S. & Soderberg-Naucler, C. Human cytomegalovirus inhibits differentiation of monocytes into dendritic cells with the consequence of depressed immunological functions. J. Virol. 77, 10943–10956 (2003).
Cresswell, P., Bangia, N., Dick, T. & Diedrich, G. The nature of the MHC class I peptide loading complex. Immunol. Rev. 172, 21–28 (1999).
Ackerman, A.L. & Cresswell, P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 5, 678–684 (2004).
Gagnon, E. et al. Endoplasmic reticulum–mediated phagocytosis is a mechanism of entry into macrophages. Cell 110, 119–131 (2002).
Houde, M. et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 (2003).
Guermonprez, P. et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
den Haan, J.M., Lehar, S.M. & Bevan, M.J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696 (2000).
Pooley, J.L., Heath, W.R. & Shortman, K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 166, 5327–5330 (2001).
Steinman, R.M. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt. Sinai J. Med. 68, 106–166 (2001).
Carbone, F.R. & Bevan, M.J. Class I-restricted processing and presentation of exogenous cell–associated antigen in vivo. J. Exp. Med. 171, 377–387 (1990).
Kovacsovics-Bankowski, M., Clark, K., Benacerraf, B. & Rock, K.L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc. Natl. Acad. Sci. USA 90, 4942–4946 (1993).
Reis e Sousa, C. & Germain, R.N. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J. Exp. Med. 182, 841–851 (1995).
Ackerman, A.L., Kyritsis, C., Tampe, R. & Cresswell, P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc. Natl. Acad. Sci. USA 100, 12889–12894 (2003).
Ahn, K. et al. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6, 613–621 (1997).
Hengel, H. et al. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6, 623–632 (1997).
Lehner, P.J., Karttunen, J.T., Wilkinson, G.W. & Cresswell, P. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing–dependent peptide translocation. Proc. Natl. Acad. Sci. USA 94, 6904–6909 (1997).
Kyritsis, C. et al. Molecular mechanism and structural aspects of transporter associated with antigen processing inhibition by the cytomegalovirus protein US6. J. Biol. Chem. 276, 48031–48039 (2001).
Hewitt, E.W., Gupta, S.S. & Lehner, P.J. The human cytomegalovirus gene product US6 inhibits ATP binding by TAP. EMBO J. 20, 387–396 (2001).
Wei, M.L. & Cresswell, P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence–derived peptides. Nature 356, 443–446 (1992).
Androlewicz, M.J. & Cresswell, P. Human transporters associated with antigen processing possess a promiscuous peptide-binding site. Immunity 1, 7–14 (1994).
Bangia, N., Lehner, P.J., Hughes, E.A., Surman, M. & Cresswell, P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur. J. Immunol. 29, 1858–1870 (1999).
Trombetta, E.S., Ebersold, M., Garrett, W., Pypaert, M. & Mellman, I. Activation of lysosomal function during dendritic cell maturation. Science 299, 1400–1403 (2003).
Constant, S.L. et al. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J. Clin. Invest. 110, 1441–1448 (2002).
Pelkmans, L., Kartenbeck, J. & Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3, 473–483 (2001).
Sandvig, K. & van Deurs, B. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 529, 49–53 (2002).
Schubert, U. et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404, 770–774 (2000).
Reits, E.A., Vos, J.C., Gromme, M. & Neefjes, J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature 404, 774–778 (2000).
Blander, J.M. & Medzhitov, R. Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014–1018 (2004).
Koller, B.H., Marrack, P., Kappler, J.W. & Smithies, O. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science 248, 1227–1230 (1990).
Pierre, P. et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 388, 787–792 (1997).
St Louis, D.C. et al. Evidence for distinct intracellular signaling pathways in CD34+ progenitor to dendritic cell differentiation from a human cell line model. J. Immunol. 162, 3237–3248 (1999).
Hebert, D.N., Foellmer, B. & Helenius, A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell 81, 425–433 (1995).
Brodsky, F.M., Bodmer, W.F. & Parham, P. Characterization of a monoclonal anti–β2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur. J. Immunol. 9, 536–545 (1979).
Dick, T.P., Bangia, N., Peaper, D.R. & Cresswell, P. Disulfide bond isomerization and the assembly of MHC class I–peptide complexes. Immunity 16, 87–98 (2002).
Berger, A.E., Davis, J.E. & Cresswell, P. Monoclonal antibody to HLA-A3. Hybridoma 1, 87–90 (1982).
Parham, P., Barnstable, C.J. & Bodmer, W.F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J. Immunol. 123, 342–349 (1979).
Porgador, A., Yewdell, J.W., Deng, Y., Bennink, J.R. & Germain, R.N. Localization, quantitation, and in situ detection of specific peptide–MHC class I complexes using a monoclonal antibody. Immunity 6, 715–726 (1997).
Hammerling, G.J., Rusch, E., Tada, N., Kimura, S. & Hammerling, U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc. Natl. Acad. Sci. USA 79, 4737–4741 (1982).
Lutz, P.M. & Cresswell, P. An epitope common to HLA class I and class II antigens, Ig light chains, and β2-microglobulin. Immunogenetics 25, 228–233 (1987).
Cannon, K.S. & Cresswell, P. Quality control of transmembrane domain assembly in the tetraspanin CD82. EMBO J. 20, 2443–2453 (2001).
Kopecky, S.A., Willingham, M.C. & Lyles, D.S. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 75, 12169–12181 (2001).
Larsson, M. et al. Efficiency of cross presentation of vaccinia virus–derived antigens by human dendritic cells. Eur. J. Immunol. 31, 3432–3442 (2001).
Ackerman, A.L. & Cresswell, P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J. Immunol. 170, 4178–4188 (2003).
Acknowledgements
Supported by the Howard Hughes Medical Institute and National Institutes of Health (F31 AI 101347 to A.L.A.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ackerman, A., Kyritsis, C., Tampé, R. et al. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol 6, 107–113 (2005). https://doi.org/10.1038/ni1147
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni1147
This article is cited by
-
Delivery of loaded MR1 monomer results in efficient ligand exchange to host MR1 and subsequent MR1T cell activation
Communications Biology (2024)
-
MARCH9‐mediated ubiquitination regulates MHC I export from the TGN
Immunology & Cell Biology (2017)
-
STIM1 promotes migration, phagosomal maturation and antigen cross-presentation in dendritic cells
Nature Communications (2017)
-
Autophagy and proteasome interconnect to coordinate cross‐presentation through MHC class I pathway in B cells
Immunology & Cell Biology (2016)
-
Antigen Cross-Presentation and Heat Shock Protein-Based Vaccines
Archivum Immunologiae et Therapiae Experimentalis (2016)