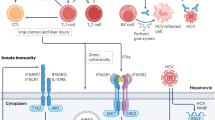
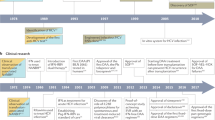
Abstract
We are entering an important new chapter in the story of hepatitis C virus (HCV) infection. There are clear challenges and opportunities. On the one hand, new HCV infections are still occurring, and an estimated 185 million people are or have previously been infected worldwide. Most HCV-infected persons are unaware of their status yet are at risk for life-threatening diseases such as cirrhosis and hepatocellular carcinoma (HCC), whose incidences are predicted to rise in the coming decade. On the other hand, new HCV infections can be prevented, and those that have already occurred can be detected and treated—viral eradication is even possible. How the story ends will largely be determined by the extent to which these rapidly advancing opportunities overcome the growing challenges and by the vigor of the public health response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hoofnagle, J.H. & Hepatitis, C. The clinical spectrum of disease. Hepatology 26, 15S–20S (1997).
Villano, S.A. et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 29, 908–914 (1999).
Cox, A.L. et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin. Infect. Dis. 40, 951–958 (2005).
Mosley, J.W. et al. Viral and host factors in early hepatitis C virus infection. Hepatology 42, 86–92 (2005).
Lee, M.H. et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J. Infect. Dis. 206, 469–477 (2012).
Bruno, S. et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology 25, 754–758 (1997).
Thomas, D.L. & Seeff, L.B. Natural history of hepatitis C. Clin. Liver Dis. 9, 383–398 (2005).
Goedert, J.J. et al. End-stage liver disease in persons with hemophilia and transfusion- associated infections. Blood 100, 1584–1589 (2002).
Thomas, D.L. et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. J. Am. Med. Assoc. 284, 450–456 (2000).
Poynard, T., Bedossa, P. & Opolon, P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 349, 825–832 (1997).
Di Bisceglie, A.M. et al. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology 14, 969–974 (1991).
Farci, P. et al. Profibrogenic chemokines and viral evolution predict rapid progression of hepatitis C to cirrhosis. Proc. Natl. Acad. Sci. USA 109, 14562–14567 (2012).
Fierer, D.S. et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J. Infect. Dis. 198, 683–686 (2008).
Agnello, V., Chung, R.T. & Kaplan, L.M. A role for hepatitis C virus infection in type II cryoglobulinemia. N. Engl. J. Med. 327, 1490–1495 (1992).
Mehta, S.H. et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann. Intern. Med. 133, 592–599 (2000).
Spiegel, B.M. et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology 41, 790–800 (2005).
Conrad, S. et al. Living with chronic hepatitis C means 'you just haven't got a normal life any more'. Chronic Illn. 2, 121–131 (2006).
Leigh, J.P. et al. Costs of hepatitis C. Arch. Intern. Med. 161, 2231–2237 (2001).
Davis, K.L. et al. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J. Clin. Gastroenterol. 45, e17–e24 (2011).
Rein, D.B. et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann. Intern. Med. 156, 263–270 (2012).
Liu, S. et al. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann. Intern. Med. 156, 279–290 (2012).
Mendel, I. et al. Detection and genotyping of the hepatitis C RNA in tear fluid from patients with chronic hepatitis C. J. Med. Virol. 51, 231–233 (1997).
Esteban, J.I. et al. High rate of infectivity and liver disease in blood donors with antibodies to hepatitis C virus. Ann. Intern. Med. 115, 443–449 (1991).
Seeff, L.B. Hepatitis C from a needlestick injury. Ann. Intern. Med. 115, 411 (1991).
Dore, G.J., Kaldor, J.M. & McCaughan, G.W. Systematic review of role of polymerase chain reaction in defining infectiousness among people infected with hepatitis C virus. Br. Med. J. 315, 333–337 (1997).
Thomas, D.L. et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. J. Infect. Dis. 177, 1480–1488 (1998).
Mast, E.E. et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J. Infect. Dis. 192, 1880–1889 (2005).
Zanetti, A.R. et al. Mother-to-infant transmission of hepatitis C virus. Lancet 345, 289–291 (1995).
Paintsil, E. et al. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J. Infect. Dis. 202, 984–990 (2010).
Doerrbecker, J. et al. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J. Infect. Dis. 207, 281–287 (2013).
Sartori, M. et al. Transmission of hepatitis C via blood splash into conjunctiva. Scand. J. Infect. Dis. 25, 270–271 (1993).
Thomas, D.L. et al. Sexual transmission of hepatitis C virus among patients attending sexually transmitted diseases clinics in Baltimore: an analysis of 309 sex partnerships. J. Infect. Dis. 171, 768–775 (1995).
Conry-Cantilena, C. et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N. Engl. J. Med. 334, 1691–1696 (1996).
Akahane, Y. et al. Hepatitis C virus infection in spouses of patients with type C chronic liver disease. Ann. Intern. Med. 120, 748–752 (1994).
van de Laar, T.J. et al. Sexual transmission of hepatitis C virus in human immunodeficiency virus-negative men who have sex with men: a series of case reports. Sex. Transm. Dis. 38, 102–104 (2011).
Terrault, N.A. et al. Sexual transmission of HCV among monogamous heterosexual couples: the HCV partners study. Hepatology 57, 881–889 (2013).
Vandelli, C. et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am. J. Gastroenterol. 99, 855–859 (2004).
Magiorkinis, G. et al. Integrating phylodynamics and epidemiology to estimate transmission diversity in viral epidemics. PLoS Comput. Biol. 9, e1002876 (2013).
Williams, I.T. et al. Incidence and transmission patterns of acute hepatitis C in the United States, 1982–2006. Arch. Intern. Med. 171, 242–248 (2011).
Hagan, H., Thiede, H. & Des Jarlais, D.C. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology 15, 543–549 (2004).
Schreiber, G.B. et al. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N. Engl. J. Med. 334, 1685–1690 (1996).
Thompson, N.D. et al. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann. Intern. Med. 150, 33–39 (2009).
Martínez-Bauer, E. et al. Hospital admission is a relevant source of hepatitis C virus acquisition in Spain. J. Hepatol. 48, 20–27 (2008).
Hauri, A.M., Armstrong, G.L. & Hutin, Y.J. The global burden of disease attributable to contaminated injections given in health care settings. Int. J. STD AIDS 15, 7–16 (2004).
Ko, Y.C. et al. Tattooing as a risk of hepatitis C virus infection. J. Med. Virol. 38, 288–291 (1992).
Mohd Hanafiah, K., Groeger, J., Flaxman, A.D. & Wiersma, S.T. et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to hepatitis C virus seroprevalence. Hepatology 57, 1333–1342 (2013).
Frank, C. et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 355, 887–891 (2000).
Strickland, G.T. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology 43, 915–922 (2006).
Guadagnino, V. et al. Hepatitis C virus infection in an endemic area of Southern Italy 14 years later: evidence for a vanishing infection. Dig. Liver Dis. 45, 403–407 (2013).
Hayashi, J. et al. Transmission of hepatitis C virus by health care workers in a rural area of Japan. Am. J. Gastroenterol. 90, 794–799 (1995).
Armstrong, G.L. et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 144, 705–714 (2006).
Baillargeon, J. et al. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health 117, 43–48 (2003).
Thomas, D.L. et al. Correlates of hepatitis C virus infections among injection drug users in Baltimore. Medicine 74, 212–220 (1995).
Perz, J.F. et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 45, 529–538 (2006).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Davis, G.L. et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138, 513–521 (2010).
Liao, S.F. et al. Fifteen-year population attributable fractions and causal pies of risk factors for newly developed hepatocellular carcinomas in 11,801 men in Taiwan. PLoS ONE 7, e34779 (2012).
El-Serag, H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1264–1273 (2012).
Tanaka, Y. et al. Molecular tracing of the global hepatitis C virus epidemic predicts regional patterns of hepatocellular carcinoma mortality. Gastroenterology 130, 703–714 (2006).
Schreiber, G.B. et al. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N. Engl. J. Med. 334, 1685–1690 (1996).
Mehta, S.H. et al. Changes in blood-borne infection risk among injection drug users. J. Infect. Dis. 203, 587–594 (2011).
McHutchison, J.G. et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339, 1485–1492 (1998).
Manns, M.P. et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358, 958–965 (2001).
Fried, M.W. et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347, 975–982 (2002).
Poordad, F. et al. Boceprevir for intreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364, 1195–1206 (2011).
Jacobson, I.M. et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364, 2405–2416 (2011).
McHutchison, J.G. et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N. Engl. J. Med. 361, 580–593 (2009).
Shiratori, Y. et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann. Intern. Med. 132, 517–524 (2000).
Veldt, B.J. et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann. Intern. Med. 147, 677–684 (2007).
Cardoso, A.C. et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J. Hepatol. 52, 652–657 (2010).
Berenguer, J. et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology 50, 407–413 (2009).
van der Meer, A.J. et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. J. Am. Med. Assoc. 308, 2584–2593 (2012).
Backus, L.I. et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin. Gastroenterol. Hepatol. 9, 509–516 (2011).
Chou, R. et al. Screening for hepatitis C virus infection in adults: a systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 158, 101–108 (2013).
Bernstein, D. et al. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology 35, 704–708 (2002).
John-Baptiste, A.A. et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am. J. Gastroenterol. 104, 2439–2448 (2009).
Liang, J. Nat. Med. 19, aaa–bbb (2013).
Patel, P.R. et al. Hepatitis C virus infections from a contaminated radiopharmaceutical used in myocardial perfusion studies. J. Am. Med. Assoc. 296, 2005–2011 (2006).
Hagan, H. et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J. Infect. Dis. 201, 378–385 (2010).
Hagan, H. et al. Reduced risk of hepatitis B and hepatitis C among injection drug users in the Tacoma syringe exchange program. Am. J. Public Health 85, 1531–1537 (1995).
van de Laar, T. et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 136, 1609–1617 (2009).
Grebely, J. et al. Reinfection with hepatitis C virus following sustained virological response in injection drug users. J. Gastroenterol. Hepatol. 25, 1281–1284 (2010).
Cottrell, E.B., Chou, R., Wasson, N., Rahman, B. & Guise, J.-M. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 158, 109–113 (2013).
Denniston, M.M. et al. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology 55, 1652–1661 (2012).
Lettmeier, B. et al. Market uptake of new antiviral drugs for the treatment of hepatitis C. J. Hepatol. 49, 528–536 (2008).
Volk, M.L. et al. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 50, 1750–1755 (2009).
Kramer, J.R. et al. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J. Hepatol. 56, 320–325 (2012).
Shivkumar, S. et al. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Ann. Intern. Med. 157, 558–566 (2012).
Smith, B.D. et al. Hepatitis C virus testing of persons born during 1945 to 1965: recommendations from the Centers for Disease Control and Prevention. Ann. Intern. Med. 157, 817–822 (2012).
Lok, A.S. et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366, 216–224 (2012).
Poordad, F. et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N. Engl. J. Med. 368, 45–53 (2013).
Gane, E.J. et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N. Engl. J. Med. 368, 34–44 (2013).
Sulkowski, M.S. et al. High rate of sustained virologic response with the all-oral combination of daclatasvir plus sofosbuvir, with or without ribavirin in treatment-naïve patients chronically infected with genotypes 1, 2, or 3 http://www.natap.org/2012/AASLD/AASLD_06.htm (2012).
Edlin, B.R. Perspective: test and treat this silent killer. Nature 474, S18–S19 (2011).
Dowdle, W.R. & Cochi, S.L. The principles and feasibility of disease eradication. Vaccine 29 (suppl. 4), D70–D73 (2011).
Rein, D.B. . et al. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig. Liver Dis. 43, 66–72 (2011).
Wong, J.B. et al. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am. J. Public Health 90, 1562–1569 (2000).
Lehman, E.M. & Wilson, M.L. Epidemic hepatitis C virus infection in Egypt: estimates of past incidence and future morbidity and mortality. J. Viral Hepat. 16, 650–658 (2009).
Deuffic, S. et al. Modeling the hepatitis C virus epidemic in France. Hepatology 29, 1596–1601 (1999).
Mariano, A. et al. Estimating the incidence, prevalence and clinical burden of hepatitis C over time in Italy. Scand. J. Infect. Dis. 41, 689–699 (2009).
Foy, E. et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102, 2986–2991 (2005).
Barrett, S. et al. The natural course of hepatitis C virus infection after 22 years in a unique homogenous cohort: spontaneous viral clearance and chronic HCV infection. Gut 49, 423–430 (2001).
Acknowledgements
This work was supported by US National Institutes of Health grant R01 DA013324.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.L.T. has served on a scientific advisory board for Gilead and Jansen; his university has received support for his research from Merck and Gilead.
Rights and permissions
About this article
Cite this article
Thomas, D. Global control of hepatitis C: where challenge meets opportunity. Nat Med 19, 850–858 (2013). https://doi.org/10.1038/nm.3184
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nm.3184
This article is cited by
-
Antimicrobial peptides: natural or synthetic defense peptides against HBV and HCV infections
VirusDisease (2022)
-
Development of a structural epitope mimic: an idiotypic approach to HCV vaccine design
npj Vaccines (2021)
-
Increased levels of circulating IL-10 in persons recovered from hepatitis C virus (HCV) infection compared with persons with active HCV infection
BMC Research Notes (2020)
-
Crowdsourcing to promote hepatitis C testing and linkage-to-care in China: a randomized controlled trial protocol
BMC Public Health (2020)
-
The efficacy and safety of direct-acting antiviral drugs in the management of hepatitis C virus-related arthritis
Egyptian Rheumatology and Rehabilitation (2020)