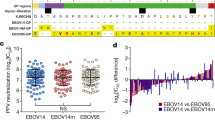
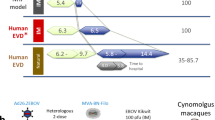
Abstract
Development of an effective vaccine against Ebola virus is of high priority. However, knowledge about potential correlates of protection and the durability of immune response after vaccination is limited. Here, we elucidate the human antibody repertoire after administration of vesicular stomatitis virus (VSV)-Ebola vaccine at 3 million, 20 million and 100 million plaque-forming units (PFU) and homologous VSV-Ebola vaccine boost in healthy adult volunteers. Whole genome-fragment phage display libraries, expressing linear and conformational epitopes of Ebola glycoprotein (GP), showed higher diversity of antibody epitopes in individuals vaccinated with 20 million PFU than in those vaccinated with 3 million or 100 million PFU. Surface plasmon resonance kinetics showed higher levels of GP-binding antibodies after a single vaccination with 20 million or 100 million PFU than with 3 million PFU, and these correlated strongly with neutralization titers. A second vaccination did not boost antibody or virus neutralization titers, which declined rapidly, and induced only minimal antibody affinity maturation. Isotype analysis revealed a predominant IgM response even after the second vaccination, which contributed substantially to virus neutralization in vitro. These findings may help identify new vaccine targets and aid development and evaluation of effective countermeasures against Ebola.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Change history
15 November 2016
In the version of this article initially published online, the authors omitted acknowledging a funding agency and researchers for providing the pseudovirion neutralization assay data. The acknowledgments section has been revised to state, “We thank the US Army Medical Research Institute of Infectious Diseases team, including S. Kwilas, M. Wisniewski and J. Hooper, for providing the pseudovirion neutralization assay data used in this study. The pseudovirion work was funded by the US Department of Defense (DoD) Medical Countermeasures Systems' Joint Vaccine Acquisition Program at Fort Detrick, Maryland. The opinions, interpretations, conclusions and recommendations contained herein are those of the authors and are not necessarily endorsed by the US DoD.” The error has been corrected for all versions of this article.
References
Centers for Disease Control and Prevention. 2014 Ebola outbreak in West Africa—case counts. Centers for Disease Control and Prevention http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html (2016).
Kanapathipillai, R. et al. Ebola vaccine--an urgent international priority. N. Engl. J. Med. 371, 2249–2251 (2014).
Henao-Restrepo, A.M. et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 386, 857–866 (2015).
Krause, P.R. Interim results from a phase 3 Ebola vaccine study in Guinea. Lancet 386, 831–833 (2015).
Marzi, A., Feldmann, F., Geisbert, T.W., Feldmann, H. & Safronetz, D. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses. Emerg. Infect. Dis. 21, 305–307 (2015).
Marzi, A. et al. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 349, 739–742 (2015).
Rampling, T. et al. A monovalent chimpanzee adenovirus Ebola vaccine—preliminary report. N. Engl. J. Med. 374, 1635–1646 (2016).
Regules, J.A. et al. A recombinant vesicular stomatitis virus Ebola vaccine—preliminary report. N. Engl. J. Med. http://dx.doi.org/10.1056/NEJMoa1414216 (2015).
Tapia, M.D. et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 16, 31–42 (2016).
Jones, S.M. et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11, 786–790 (2005).
Dye, J.M. et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc. Natl. Acad. Sci. USA 109, 5034–5039 (2012).
Holtsberg, F.W. et al. Pan-ebolavirus and pan-filovirus mouse monoclonal antibodies: protection against Ebola and Sudan viruses. J. Virol. 90, 266–278 (2016).
Marzi, A. et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc. Natl. Acad. Sci. USA 110, 1893–1898 (2013).
Wilson, J.A. et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287, 1664–1666 (2000).
Matassov, D. et al. Vaccination with a highly attenuated recombinant vesicular stomatitis virus vector protects against challenge with a lethal dose of Ebola virus. J. Infect. Dis. 212 (Suppl. 2), S443–S451 (2015).
Blaney, J.E. et al. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathog. 9, e1003389 (2013).
Wong, G. et al. Immune parameters correlate with protection against Ebola virus infection in rodents and nonhuman primates. Sci. Transl. Med. 4, 158ra146 (2012).
Ruibal, P. et al. Unique human immune signature of Ebola virus disease in Guinea. Nature 533, 100–104 (2016).
Cohen, J. & Enserink, M. Clinical trials. Ebola vaccines face daunting path to approval. Science 349, 1272–1273 (2015).
Krause, P.R., Cavaleri, M., Coleman, G. & Gruber, M.F. Approaches to demonstration of Ebola virus vaccine efficacy. Lancet Infect. Dis. 15, 627–629 (2015).
Khurana, S. et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2, 15ra5 (2010).
Khurana, S. et al. Antigenic fingerprinting of H5N1 avian influenza using convalescent sera and monoclonal antibodies reveals potential vaccine and diagnostic targets. PLoS Med. 6, e1000049 (2009).
Khurana, S. et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 3, 85ra48 (2011).
Flyak, A.I. et al. Cross-reactive and potent neutralizing antibody responses in human survivors of natural Ebolavirus infection. Cell 164, 392–405 (2016).
Davidson, E. et al. Mechanism of binding to Ebola virus glycoprotein by the ZMapp, ZMAb, and MB-003 cocktail antibodies. J. Virol. 89, 10982–10992 (2015).
Murin, C.D. et al. Structures of protective antibodies reveal sites of vulnerability on Ebola virus. Proc. Natl. Acad. Sci. USA 111, 17182–17187 (2014).
Lee, J.E. et al. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 454, 177–182 (2008).
Yang, J. et al. The I-TASSER Suite: protein structure and function prediction. Nat. Methods 12, 7–8 (2015).
Geisbert, T.W. et al. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of Ebola virus. J. Virol. 83, 7296–7304 (2009).
Chung, K.Y. et al. ISCOMATRIX™ adjuvant promotes epitope spreading and antibody affinity maturation of influenza A H7N9 virus-like particle vaccine that correlate with virus neutralization in humans. Vaccine 33, 3953–3962 (2015).
Nicholson, K.G. et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357, 1937–1943 (2001).
Jackson, L.A. et al. Effect of varying doses of a monovalent H7N9 influenza vaccine with and without AS03 and MF59 adjuvants on immune response: a randomized clinical trial. J. Am. Med. Assoc. 314, 237–246 (2015).
Mulligan, M.J. et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. J. Am. Med. Assoc. 312, 1409–1419 (2014).
Fuentes, S., Coyle, E.M., Beeler, J., Golding, H. & Khurana, S. Antigenic fingerprinting following primary RSV infection in young children identifies novel antigenic sites and reveals unlinked evolution of human antibody repertoires to fusion and attachment glycoproteins. PLoS Pathog. 12, e1005554 (2016).
Gitlin, A.D. et al. Humoral immunity. T cell help controls the speed of the cell cycle in germinal center B cells. Science 349, 643–646 (2015).
McHeyzer-Williams, L.J., Milpied, P.J., Okitsu, S.L. & McHeyzer-Williams, M.G. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat. Immunol. 16, 296–305 (2015).
Khurana, S. et al. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. J. Infect. Dis. 208, 413–417 (2013).
Ledgerwood, J.E. et al. Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect. J. Infect. Dis. 208, 418–422 (2013).
Halliley, J.L. et al. High-affinity H7 head and stalk domain-specific antibody responses to an inactivated influenza H7N7 vaccine after priming with live attenuated influenza vaccine. J. Infect. Dis. 212, 1270–1278 (2015).
Talaat, K.R. et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J. Infect. Dis. 209, 1860–1869 (2014).
Acknowledgements
We thank S. Rubin and H. Golding for reviewing the manuscript and J. Voell, P. Munoz, R. McConnell, H. Baus, C. Rehm and J. Metcalf for help with clinical studies. We thank J. Crowe (Vanderbilt University) for the gift of MAb 289 and MAb 324. The clinical trial from which the test sera were derived was funded partly by the US National Institute of Allergy and Infectious Diseases. The antibody characterization work described in this manuscript was supported by FDA-MCMi-Ebola funds to S.K. The latter funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. This project was funded in whole or in part with federal funds from the US National Cancer Institute under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government. We thank the US Army Medical Research Institute of Infectious Diseases team, including S. Kwilas, M. Wisniewski and J. Hooper, for providing the pseudovirion neutralization assay data used in this study. The pseudovirion work was funded by the US Department of Defense (DoD) Medical Countermeasures Systems' Joint Vaccine Acquisition Program at Fort Detrick, Maryland. The opinions, interpretations, conclusions and recommendations contained herein are those of the authors and are not necessarily endorsed by the US DoD.
Author information
Authors and Affiliations
Contributions
S.K. designed research; S.F., S.R., E.M.C. and S.K. performed research; J.H.B. and R.T.D. conducted phase 1 study and contributed clinical samples; S.K., J.H.B. and R.T.D. contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Tables, Figures and Text
Supplementary Tables 1–3 and Supplementary Figures 1–10 (PDF 979 kb)
Rights and permissions
About this article
Cite this article
Khurana, S., Fuentes, S., Coyle, E. et al. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med 22, 1439–1447 (2016). https://doi.org/10.1038/nm.4201
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nm.4201
This article is cited by
-
Antibody affinity and cross-variant neutralization of SARS-CoV-2 Omicron BA.1, BA.2 and BA.3 following third mRNA vaccination
Nature Communications (2022)
-
The use of viral vectors in vaccine development
npj Vaccines (2022)
-
SARS-CoV-2 immune repertoire in MIS-C and pediatric COVID-19
Nature Immunology (2021)
-
Antibody affinity maturation and plasma IgA associate with clinical outcome in hospitalized COVID-19 patients
Nature Communications (2021)
-
Polyclonal and convergent antibody response to Ebola virus vaccine rVSV-ZEBOV
Nature Medicine (2019)