Abstract
Lung-innervating nociceptor sensory neurons detect noxious or harmful stimuli and consequently protect organisms by mediating coughing, pain, and bronchoconstriction. However, the role of sensory neurons in pulmonary host defense is unclear. Here, we found that TRPV1+ nociceptors suppressed protective immunity against lethal Staphylococcus aureus pneumonia. Targeted TRPV1+-neuron ablation increased survival, cytokine induction, and lung bacterial clearance. Nociceptors suppressed the recruitment and surveillance of neutrophils, and altered lung γδ T cell numbers, which are necessary for immunity. Vagal ganglia TRPV1+ afferents mediated immunosuppression through release of the neuropeptide calcitonin gene–related peptide (CGRP). Targeting neuroimmunological signaling may be an effective approach to treat lung infections and bacterial pneumonia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
16 July 2018
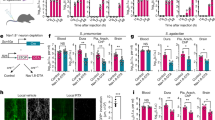
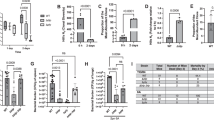
In the version of this article initially published, the line graph showing TNF-α levels in Fig. 2d was inadvertently duplicated. A graph of IL-6 levels should be shown in place of the duplication. These results were also incorrectly described in the main text, which originally stated: "At an early time point of infection (6 h), RTX-treated mice showed higher induction of total inflammatory-protein levels in the bronchoalveolar lavage fluid (BALF) (Fig. 2c), as well as levels of the cytokines TNF-α and IL-6, and the chemokine CXCL-1 (Fig. 2d)". This should instead read: "At an early time point of infection (6 h), RTX-treated mice showed higher induction of total inflammatory-protein levels in the bronchoalveolar lavage fluid (BALF) (Fig. 2c), as well as levels of the cytokine TNF-α and the chemokine CXCL-1 (Fig. 2d)". In the supplementary information initially posted online, incorrect bar graphs were presented in Supplementary Fig. 1b (VG, TRPV1+ data, top panel) and Supplementary Fig. 4b (DRG, CGRP+ data, middle panel). The errors have been corrected in the HTML and PDF versions of this article.
References
Parker, D. & Prince, A. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin. Immunopathol. 34, 281–297 (2012).
Tong, S.Y., Davis, J.S., Eichenberger, E., Holland, T.L. & Fowler, V.G. Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 28, 603–661 (2015).
Inoshima, I. et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 17, 1310–1314 (2011).
Mizgerd, J.P. Acute lower respiratory tract infection. N. Engl. J. Med. 358, 716–727 (2008).
Tränkner, D., Hahne, N., Sugino, K., Hoon, M.A. & Zuker, C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc. Natl. Acad. Sci. USA 111, 11515–11520 (2014).
Chang, R.B., Strochlic, D.E., Williams, E.K., Umans, B.D. & Liberles, S.D. Vagal sensory neuron subtypes that differentially control breathing. Cell 161, 622–633 (2015).
Mazzone, S.B. & Undem, B.J. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 96, 975–1024 (2016).
Basbaum, A.I., Bautista, D.M., Scherrer, G. & Julius, D. Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009).
Canning, B.J., Mori, N. & Mazzone, S.B. Vagal afferent nerves regulating the cough reflex. Respir. Physiol. Neurobiol. 152, 223–242 (2006).
Dubin, A.E. & Patapoutian, A. Nociceptors: the sensors of the pain pathway. J. Clin. Invest. 120, 3760–3772 (2010).
Caceres, A.I. et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc. Natl. Acad. Sci. USA 106, 9099–9104 (2009).
Talbot, S. et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87, 341–354 (2015).
Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 29, 355–384 (2013).
Cavanaugh, D.J. et al. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 31, 10119–10127 (2011).
Mishra, S.K. & Hoon, M.A. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol. Cell. Neurosci. 43, 157–163 (2010).
Pogorzala, L.A., Mishra, S.K. & Hoon, M.A. The cellular code for mammalian thermosensation. J. Neurosci. 33, 5533–5541 (2013).
Mishra, S.K., Tisel, S.M., Orestes, P., Bhangoo, S.K. & Hoon, M.A. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 30, 582–593 (2011).
Kissin, I. & Szallasi, A. Therapeutic targeting of TRPV1 by resiniferatoxin, from preclinical studies to clinical trials. Curr. Top. Med. Chem. 11, 2159–2170 (2011).
Kashem, S.W. et al. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43, 515–526 (2015).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Abrahamsen, B. et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321, 702–705 (2008).
Fernandes, E.S. et al. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 188, 5741–5751 (2012).
Rigby, K.M. & DeLeo, F.R. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 34, 237–259 (2012).
Yipp, B.G. et al. The lung is a host defense niche for immediate neutrophil-mediated vascular protection. Sci. Immunol. 2, eaam8929 (2017).
Yipp, B.G. et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 (2012).
Su, A.I. et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101, 6062–6067 (2004).
Zheng, J., Liu, Y., Lau, Y.L. & Tu, W. γδ-T cells: an unpolished sword in human anti-infection immunity. Cell. Mol. Immunol. 10, 50–57 (2013).
Murphy, A.G. et al. Staphylococcus aureus infection of mice expands a population of memory γδ T cells that are protective against subsequent infection. J. Immunol. 192, 3697–3708 (2014).
Pinho-Ribeiro, F.A., Verri, W.A. Jr. & Chiu, I.M. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 38, 5–19 (2017).
Chiu, I.M. et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57 (2013).
Cheung, G.Y., Wang, R., Khan, B.A., Sturdevant, D.E. & Otto, M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 79, 1927–1935 (2011).
Shields, S.D. et al. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 153, 2017–2030 (2012).
Veiga-Fernandes, H. & Mucida, D. Neuro-immune interactions at barrier surfaces. Cell 165, 801–811 (2016).
Liu, B. et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 113, E7572–E7579 (2016).
Gabanyi, I. et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016).
Tay, S.S., Roediger, B., Tong, P.L., Tikoo, S. & Weninger, W. The skin-resident immune network. Curr. Dermatol. Rep. 3, 13–22 (2013).
Cho, J.S. et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 (2010).
Cheng, M. & Hu, S. Lung-resident γδ T cells and their roles in lung diseases. Immunology 151, 375–384 (2017).
Williams, E.K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Cardoso, V. et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549, 277–281 (2017).
Klose, C.S.N. et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017).
Wallrapp, A. et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549, 351–356 (2017).
Franco-Cereceda, A. et al. Cardiovascular effects of calcitonin gene-related peptides I and II in man. Circ. Res. 60, 393–397 (1987).
Sawant, K.V. et al. Chemokine CXCL1-mediated neutrophil trafficking in the lung: role of CXCR2 activation. J. Innate Immun. 7, 647–658 (2015).
Kwon, M.J. et al. CCL2 mediates neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J. Neurosci. 35, 15934–15947 (2015).
Guan, Z. et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 19, 94–101 (2016).
Pavlov, V.A. et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 23, 41–45 (2009).
de Jong, M.D. et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207 (2006).
Tisoncik, J.R. et al. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76, 16–32 (2012).
Hommes, T.J. et al. DNAX-activating protein of 12 kDa impairs host defense in pneumococcal pneumonia. Crit. Care Med. 42, e783–e790 (2014).
Xiong, H. et al. Innate lymphocyte/Ly6Chi monocyte crosstalk promotes Klebsiella Pneumoniae clearance. Cell 165, 679–689 (2016).
Labandeira-Rey, M. et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315, 1130–1133 (2007).
Wang, R. et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514 (2007).
Ghasemlou, N., Chiu, I.M., Julien, J.P. & Woolf, C.J. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. USA 112, E6808–E6817 (2015).
Heng, T.S., Painter, M.W. & The Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008).
Acknowledgements
We thank K. Blake and N. Lai for technical assistance, and J. Bubeck Wardenburg and C. Altier for helpful discussions. We thank M. Hoon (NIH) for Trpv1-Dtr mice, C. Benoist (Harvard Medical School) and A. Mann (Harvard Medical School) for Tcrd−/− mice, G. Pier (Brigham and Women's Hospital) and M. Gadjeva (Brigham and Women's Hospital) for P. aeruginosa, R. Malley (Boston Children's Hospital) for S. pneumoniae, and M. Otto (NIH) for S. aureus bacterial strains. This work was supported by National Institutes of Health (NIH) grants DP2AT009499 (I.M.C.), K22AI114810 (I.M.C.), R01AI130019 (I.M.C.), 1KO8AI123516 (P.R.B.), and R01HL132255 (S.D.L.); an HHMI Faculty Scholars Award (S.D.L.); NIH 5F31HL132645 (B.D.U.); and Canadian Institutes of Health Research (CIHR) grant RS-342013 (B.G.Y.).
Author information
Authors and Affiliations
Contributions
P.B. and I.M.C. conceived the study. P.B., B.D.U., L.L., B.G.Y., A.W., P.R.B., V.K.K., S.D.L., and I.M.C. designed experiments, analyzed data, and interpreted results. P.B. performed animal infections, survival analysis, bacterial-load recovery, cytokine measurements, FACS, CGRP assays, and neutrophil killing experiments; B.D.U. performed VG injections and immunostaining; A.W. and P.R.B. performed purification of lung cells and quantitative PCR experiments; M.B. performed lung immunostaining and quantification; T.K. and Y.W. performed cytokine measurements and histology; Y.Z. performed neuronal cultures and CGRP assays; L.L. and B.G.Y. performed animal infections with in vivo intravital microscopy of neutrophil movement and migration. P.B. and I.M.C. wrote the manuscript, which was edited by S.D.L., B.G.Y., B.D.U., P.B. and I.M.C.
Corresponding author
Ethics declarations
Competing interests
P.B. and I.M.C. are co-inventors on a patent application filed by Harvard incorporating discoveries described in the manuscript.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–33 (PDF 13453 kb)
Neutrophil dynamic behaviors revealed using pulmonary intravital microscopy
Mice were pretreated with either vehicle control or RTX 4 weeks prior to GFP-S. aureus (green) pneumonia. Animals received 1x108 CFU into the lung and intravital imaging was performed at 4 hours post-infection. Neutrophils were visualized using fluorescently conjugated anti-Ly6G antibodies (red) and the endothelium was visualized using fluorescently conjugated anti-CD31 antibodies given intravenously prior to imaging. Representative video recordings of experiments from vehicle control and RTX-treated mice are shown side by side for comparison and then individually. Imaging experiments were repeated 3 separate times for vehicle control and 4 times for RTX. (MOV 816 kb)
Rights and permissions
About this article
Cite this article
Baral, P., Umans, B., Li, L. et al. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat Med 24, 417–426 (2018). https://doi.org/10.1038/nm.4501
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nm.4501
This article is cited by
-
Gut sensory neurons as regulators of neuro-immune-microbial interactions: from molecular mechanisms to precision therapy for IBD/IBS
Journal of Neuroinflammation (2025)
-
Nociceptor neurons promote PDAC progression and cancer pain by interaction with cancer-associated fibroblasts and suppression of natural killer cells
Cell Research (2025)
-
Prospective multicenter study identifying prognostic biomarkers and microbial profiles in severe CAP using BALF, blood mNGS, and PBMC transcriptomics
Scientific Reports (2025)
-
Neuro-immune cross-talk in cancer
Nature Reviews Cancer (2025)
-
Psychological stress-induced ALKBH5 deficiency promotes tumour innervation and pancreatic cancer via extracellular vesicle transfer of RNA
Nature Cell Biology (2025)