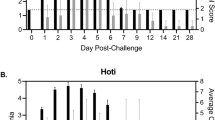
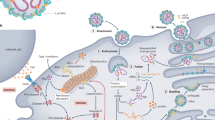
Abstract
The agents causing viral hemorrhagic fever (VHF) are a taxonomically diverse group of viruses that may share commonalities in the process whereby they produce systemic and frequently fatal disease. Significant progress has been made in understanding the biology of the Ebola virus, one of the best known examples. This knowledge has guided our thinking about other VHF agents, including Marburg, Lassa, the South American arenaviruses, yellow fever, Crimean-Congo and Rift Valley fever viruses. Comparisons among VHFs show that a common pathogenic feature is their ability to disable the host immune response by attacking and manipulating the cells that initiate the antiviral response. Of equal importance, these comparisons highlight critical gaps in our knowledge of these pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Geisbert, T.W., Pushko, P., Anderson, K., Smith, J. & Davis, K.J. & Jahrling, P.B. Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg. Infect. Dis. 8, 503–507 (2002).
Walker, D.H. & Murphy, F.A. Pathology and pathogenesis of arenavirus infections. Curr. Top. Microbiol. Immunol. 133, 89–113 (1987).
WHO. Ebola haemorrhagic fever in Sudan, 1976. Report of an international study team. Bull. World Health Organ. 56, 247–270 (1978).
WHO. Ebola haemorrhagic fever in Zaire, 1976. Report of an international commission. Bull. World Health Organ. 56, 271–293 (1978).
Khan, A.S. et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. J. Infect. Dis. 179 Suppl 1, S76–S86 (1999).
Fisher-Hoch, S.P. et al. Physiological and immunologic disturbances associated with shock in a primate model of Lassa fever. J. Infect. Dis. 155, 465–474 (1987).
Morrill, J.C. et al. Pathogenesis of Rift Valley fever in rhesus monkeys: role of the interferon response. Arch. Virol. 110, 195–212 (1990).
Fisher-Hoch, S.P. et al. Haematological and biochemical monitoring of Ebola infection in rhesus monkeys: implications for patient management. Lancet 2, 1055–1058 (1983).
Fisher-Hoch, S.P. et al. Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola). J. Infect. Dis. 152, 887–894 (1985).
Jaax, N.K. et al. Lethal experimental infection of rhesus monkeys with Ebola-Zaire (Mayinga) virus by the oral and conjunctival route of exposure. Arch. Pathol. Lab. Med. 120, 140–155 (1996).
Geisbert, T.W. et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am. J. Pathol. 163, 2347–2370 (2003).
Elsner, B., Schwarz, E., Mando, O.G., Maiztegui, J. & Vilches, A. Pathology of 12 fatal cases of Argentine hemorrhagic fever. Am. J. Trop. Med. Hyg. 22, 229–236 (1973).
McKee, K.T., Jr, Mahlandt, B.G., Maiztegui, J.I., Green, D.E. & Peters, C.J. Virus-specific factors in Argentine hemorrhagic fever in rhesus macaques. J. Med. Virol. 22, 99–111 (1987).
Monath, T.P. & Barrett, A.D. Pathogenesis and pathophysiology of yellow fever. Adv. Virus Res. 60, 343–395 (2003).
Maiztegui, J.I. et al. Ultrastructural and immunohistochemical studies in five cases of Argentine hemorrhagic fever. J. Infect. Dis. 132, 35–53 (1975).
Jahrling, P.B. et al. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J. Infect. Dis. 141, 580–589 (1980).
Gonzalez, P.H., Cossio, P.M., Arana, R., Maiztegui, J.I. & Laugens, R.P. Lymphatic tissue in Argentine hemorrhagic fever. Pathologic features. Arch. Pathol. Lab. Med. 104, 250–254 (1980).
De Brito, T. et al. Human fatal yellow fever. Immunohistochemical detection of viral antigens in the liver, kidney and heart. Pathol. Res. Pract. 188, 177–181 (1992).
Geisbert, T.W., Jahrling, P.B., Hanes, M.A. & Zack, P.M. Association of Ebola-related Reston virus particles and antigen with tissue lesions of monkeys imported to the United States. J. Comp. Pathol. 106, 137–152 (1992).
Burt, F.J. et al. Immunohistochemical and in situ localization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch. Pathol. Lab. Med. 121, 839–846 (1997).
Geisbert, T.W. & Jaax, N.K. Marburg hemorrhagic fever: report of a case studied by immunohistochemistry and electron microscopy. Ultrastruct. Pathol. 22, 3–17 (1998).
Zaki, S.R. & Goldsmith, C.S. Pathologic features of filovirus infections in humans. Curr. Top. Microbiol. Immunol. 235, 97–116 (1999).
de Filippis, A.M. et al. Outbreak of jaundice and hemorrhagic fever in the Southeast of Brazil in 2001: detection and molecular characterization of yellow fever virus. J. Med. Virol. 68, 620–627 (2002).
Geisbert, T.W. et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am. J. Pathol. 163, 2371–2382 (2003).
Becker, S., Spiess, M. & Klenk, H.D. The asialoglycoprotein receptor is a potential liver-specific receptor for Marburg virus. J. Gen. Virol. 76, 393–399 (1995).
Chan, S.Y. et al. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 106, 117–126 (2001).
Alvarez, C.P. et al. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76, 6841–6844 (2002).
Simmons, G. et al. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305, 115–123 (2003).
Marzi, A. et al. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 78, 12090–12095 (2004).
Takada, A. et al. Downregulation of beta1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology 278, 20–26 (2000).
Takada, A. et al. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 78, 2943–2947 (2004).
Cao, W. et al. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282, 2079–2081 (1998).
Spiropoulou, C.F., Kunz, S., Rollin, P.E., Campbell, K.P. & Oldstone, M.B. New World arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J. Virol. 76, 5140–5146 (2002).
Gallaher, W.R., DiSimone, C. & Buchmeier, M.J. The viral transmembrane superfamily: possible divergence of Arenavirus and Filovirus glycoproteins from a common RNA virus ancestor. BMC Microbiol. 1, http://www.biomedcentral.com/1471-2180/1/1 (2001).
Yang, Z. et al. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6, 886–889 (2000).
Lukashevich, I.S. et al. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-alpha gene expression. J. Med. Virol. 59, 552–560 (1999).
Gear, J.S. et al. Outbreake of Marburg virus disease in Johannesburg. Br. Med. J. 4, 489–493 (1975).
Murphy, F.A. Pathology of Ebola virus infection, in Ebola Virus Haemorrhagic Fever (ed Pattyn, S.R.) (Elsevier/North-Holland Biomedical Press, New York, 1978).
Terrell, T.G., Stookey, J.L., Eddy, G.A. & Kastello, M.D. Pathology of Bolivian hemorrhagic fever in the rhesus monkey. Am. J. Pathol. 73, 477–494 (1973).
Feldmann, H. et al. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J. Virol. 70, 2208–2214 (1996).
Callis, R.T., Jahrling, P.B. & DePaoli, A. Pathology of Lassa virus infection in the rhesus monkey. Am. J. Trop. Med. Hyg. 31, 1038–1045 (1982).
Frame, J.D. Clinical features of Lassa fever in Liberia. Rev. Infect. Dis. 11 Suppl 4, S783–S789 (1989).
Child, P.L., MacKenzie, R.B., Valverde, L.R. & Johnson, K.M. Bolivian hemorrhagic fever: a pathological description. Arch. Pathol. 83, 434–445 (1967).
Gedigk, P., Bechtelsheimer, H. & Korb, G. Pathologic anatomy of the Marburg virus disease, in Marburg Virus Disease (eds Martini, G.A. & Siegert, R.) (Springer-Verlag, New York, 1971).
Winn, W.C., Jr & Walker, D.H. The pathology of human Lassa fever. Bull. World Health Organ. 52, 535–545 (1975).
Walker, D.H. et al. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 107, 349–356 (1982).
Green, D.E., Mahlandt, B.G. & McKee, K.T., Jr. Experimental Argentine hemorrhagic fever in rhesus macaques: virus-specific variations in pathology. J. Med. Virol. 22, 113–133 (1987).
Elton, N.W., Romero, A. & Trejos, A. Clinical pathology of yellow fever. Am. J. Clin. Pathol. 25, 135–146 (1955).
Martini, G.A. Marburg virus disease: clinical syndrome, in Marburg Virus Disease (eds Martini, G.A. & Siegert, R.) (Springer-Verlag, New York, 1971).
Joubert, J.R., King, J.B., Rossouw, D.J. & Cooper, R. A nosocomial outbreak of Crimean-Congo haemorrhagic fever at Tygerberg Hospital. Part III. Clinical pathology and pathogenesis. S. Afr. Med. J. 68, 722–728 (1985).
Peters, C.J., Jahrling, P.B., Liu, C.T., Kenyon, R.H. & McKee, K.T., Jr. & Barrera Oro, J.G. Experimental studies of arenaviral hemorrhagic fevers. Curr. Top. Microbiol. Immunol. 134, 5–68 (1987).
Peters, C.J. et al. Experimental Rift Valley fever in rhesus macaques. Arch. Virol. 99, 31–44 (1988).
Fisher-Hoch, S.P., McCormick, J.B., Sasso, D. & Craven, R.B. Hematologic dysfunction in Lassa fever. J. Med. Virol. 26, 127–135 (1988).
Swanepoel, R. et al. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 11 Suppl 4, S794–S800 (1989).
Fisher-Hoch, S.P. et al. Pathogenic potential of filoviruses: role of geographic origin of primate host and virus strain. J. Infect. Dis. 166, 753–763 (1992).
Sanchez, A. et al. Filoviridae: Marburg and Ebola viruses, in Fields Virology (eds Knipe, D.S. & Howley, P.M.) (Lippincott Williams & Wilkins, Philadelphia, 2001).
Schmitz, H. et al. Monitoring of clinical and laboratory data in two cases of imported Lassa fever. Microbes Infect. 4, 43–50 (2002).
Ergonul, O. et al. Characteristics of patients with Crimean-Congo hemorrhagic fever in a recent outbreak in Turkey and impact of oral ribavirin therapy. Clin. Infect. Dis. 39, 284–287 (2004).
McLeod, C.G., Jr, Stookey, J.L., White, J.D., Eddy, G.A. & Fry, G.A. Pathology of Bolivian Hemorrhagic fever in the African green monkey. Am. J. Trop. Med. Hyg. 27, 822–826 (1978).
Peters, C.J. Arenaviruses, in Clincal Virology (Eds Richman, D.D., Whitley, R.J. & Hayden, F.G.) (Churchill Livingstone, New York, 1997).
Hudson, N. The pathology of experimental yellow fever in the Macaca rhesus. III. Comparison with the pathology of yellow fever in man. Am. J. Pathol. 4, 419–439 (1928).
Klotz, O. & Belt, T.H. Pathology in spleen in yellow fever. Am. J. Pathol. 6, 655–662 (1930).
Zlotnik, I. Marburg agent disease: Pathology. Trans. R. Soc. Trop. Med. Hyg. 63, 310–323 (1969).
Murphy, F.A., Simpson, D.I.H., Whitfield, S.G., Zlotnik, I. & Carter, G.B. Marburg virus infection in monkeys. Lab. Invest. 24, 279–291 (1971).
Edington, G.M. & White, H.A. The pathology of Lassa fever. Trans. R. Soc. Trop. Med. Hyg. 66, 381–389 (1972).
Monath, T.P., Brinker, K.R., Chandler, F.W., Kemp, G.E. & Cropp, C.B. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am. J. Trop. Med. Hyg. 30, 431–443 (1981).
Baskerville, A., Satti, A., Murphy, F.A. & Simpson, D.I. Congo-Crimean haemorrhagic fever in Dubai: histopathological studies. J. Clin. Pathol. 34, 871–874 (1981).
Simpson, D.I.H. Marburg agent disease: in monkeys. Trans. R. Soc. Trop. Med. Hyg. 63, 303–309 (1969).
Havemann, K. & Schmidt, H.A. Haematological findings in Marburg virus disease: evidence for involvement of the immunological system, in Marburg Virus Disease (eds Martini, G.A. & Siegert, R.) (Springer-Verlag, New York, 1971).
Kastello, M.D., Eddy, G.A. & Kuehne, R.W. A rhesus monkey model for the study of Bolivian hemorrhagic fever. J. Infect. Dis. 133, 57–62 (1976).
Wagner, F.S., Eddy, G.A. & Brand, O.M. The African green monkey as an alternate primate host for studying Machupo virus infection. Am. J. Trop. Med. Hyg. 26, 159–162 (1977).
Fisher-Hoch, S.P. et al. Protection of rhesus monkeys from fatal Lassa fever by vaccination with a recombinant vaccinia virus containing the Lassa virus glycoprotein gene. Proc. Natl. Acad. Sci. USA 86, 317–321 (1989).
Vallejos, D.A., Ambrosio, A.M., Feuillade, M.R. & Maiztegui, J.I. Lymphocyte subsets alteration in patients with Argentine hemorrhagic fever. J. Med. Virol. 27, 160–163 (1989).
Sanchez, A. et al. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J. Virol. 78, 10370–10377 (2004).
Baize, S. et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5, 423–426 (1999).
Geisbert, T.W. et al. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab. Invest. 80, 171–186 (2000).
Reed, D.S., Hensley, L.E., Geisbert, J.B., Jahrling, P.B. & Geisbert, T.W. Depletion of peripheral blood T lymphocytes and NK cells during the course of Ebola hemorrhagic fever in cynomolgus macaques. Viral Immunol. 17, 390–400 (2004).
Hensley, L.E., Young, H.A., Jahrling, P.B. & Geisbert, T.W. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol. Lett. 80, 169–179 (2002).
Takabayashi, A. et al. Nitric oxide induces a decrease in the mitochondrial membrane potential of peripheral blood lymphocytes, especially in natural killer cells. Antioxid. Redox Signal. 2, 673–680 (2000).
Mahanty, S. et al. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J. Immunol. 170, 2797–2801 (2003).
Daniel, P.T., Scholz, C., Westermann, J., Dorken, B. & Pezzutto, A. Dendritic cells prevent CD95 mediated T lymphocyte death through costimulatory signals. Adv. Exp. Med. Biol. 451, 173–177 (1998).
Baize, S. et al. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172, 2861–2869 (2004).
Raftery, M.J. et al. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15, 997–1009 (2001).
Volchkov, V.E., Blinov, V.M. & Netesov, S.V. The envelope glycoprotein of Ebola virus contains an immunosuppressive-like domain similar to oncogenic retroviruses. FEBS Lett. 305, 181–184 (1992).
Bukreyev, A., Volchkov, V.E., Blinov, V.M. & Netesov, S.V. The GP-protein of Marburg virus contains the region similar to the 'immunosuppressive domain' of oncogenic retrovirus P15E proteins. FEBS Lett. 323, 183–187 (1993).
Calandra, T., Gerain, J., Heumann, D. & Baumgartner, J.D. & Glauser MP. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines. The Swiss-Dutch J5 Immunoglobulin Study Group. Am. J. Med. 91, 23–29 (1991).
Damas, P. et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann. Surg. 215, 356–362 (1992).
Marty, C. et al. Circulating interleukin-8 concentrations in patients with multiple organ failure of septic and nonseptic origin. Crit. Care Med. 22, 673–679 (1994).
Levis, S.C. et al. Endogenous interferon in Argentine hemorrhagic fever. J. Infect. Dis. 149, 428–433 (1984).
Levis, S.C. et al. Correlation between endogenous interferon and the clinical evolution of patients with Argentine hemorrhagic fever. J. Interferon Res. 5, 383–389 (1985).
Heller, M.V., Saavedra, M.C., Falcoff, R., Maiztegui, J.I. & Molinas, F.C. Increased tumor necrosis factor-alpha levels in Argentine hemorrhagic fever. J. Infect. Dis. 166, 1203–1204 (1992).
Marta, R.F. et al. Proinflammatory cytokines and elastase-α-1-antitrypsin in Argentine hemorrhagic fever. Am. J. Trop. Med. Hyg. 60, 85–89 (1999).
Villinger, F. et al. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J. Infect. Dis. 179 Suppl 1, S188–S191 (1999).
Ignatiev, G.M., Dadaeva, A.A., Luchko, S.V. & Chepurnov, A.A. Immune and pathophysiological processes in baboons experimentally infected with Ebola virus adapted to guinea pigs. Immunol. Lett. 71, 131–140 (2000).
Mahanty, S. et al. Low levels of interleukin-8 and interferon-inducible protein-10 in serum are associated with fatal infections in acute Lassa fever. J. Infect. Dis. 183, 1713–1721 (2001).
Ter Meulen, J. et al. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J. Infect. Dis. 190, 1821–1827 (2004).
Stroher, U. et al. Infection and activation of monocytes by Marburg and Ebola viruses. J. Virol. 75, 11025–11033 (2001).
Gomez, R.M. et al. Endothelial cell function alteration after Junin virus infection. Thromb. Haemost. 90, 326–333 (2003).
Leroy, E.M. et al. Human asymptomatic Ebola infection and strong inflammatory response. Lancet 355, 2210–2215 (2000).
Peters, C.J., Liu, C.T., Anderson, G.W., Jr, Morrill, J.C. & Jahrling, P.B. Pathogenesis of viral hemorrhagic fevers: Rift Valley fever and Lassa fever contrasted. Rev. Infect. Dis. 11 Suppl 4, S743–S749 (1989).
Asper, M. et al. Inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus. J. Virol. 78, 3162–3169 (2004).
Foster, G.R. & Finter, N.B. Are all type I human interferons equivalent? J. Viral Hepat. 5, 143–152 (1998).
Basler, C.F. et al. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97, 12289–12294 (2000).
Basler, C.F. et al. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77, 7945–7956 (2003).
Basler, C.F. & Palese, P. Modulation of innate immunity by filoviruses, in Ebola and Marburg viruses: molecular and cellular biology (eds Klenk, H.D. & Feldmann, H) (Horizon Bioscience, Norfolk, UK, 2004).
Bouloy, M. et al. Genetic evidence for an interferon-antagonist function of rift valley fever virus nonstructural protein NSs. J. Virol. 75, 1371–1377 (2001).
Li, H. & Forstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 190, 244–254 (2000).
Mammen, E.F. Disseminated intravascular coagulation (DIC). Clin. Lab. Sci. 13, 239–245 (2000).
Levi, M. Current understanding of disseminated intravascular coagulation. Br. J. Haematol. 124, 567–576 (2004).
Martini, G.A., Knauff, H.G., Schmidt, H.A., Mayer, G. & Baltzer, G. A hitherto unknown infectious disease contracted from monkeys. “Marburg-virus” disease. Ger. Med. Mon. 13, 457–470 (1968).
Bwaka, M.A. et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J. Infect. Dis. 179 suppl. Suppl 1, S1–S7 (1999).
Bowen, E.T., Platt, G.S., Simpson, D.I., McArdell, L.B. & Raymond, R.T. Ebola haemorrhagic fever: experimental infection of monkeys. Trans. R. Soc. Trop. Med. Hyg. 72, 188–191 (1978).
Geisbert, T.W. et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet 362, 1953–1958 (2003).
Mackenzie, R.B., Beye, H.K. & Valverde, L. & Garr'on, H. Epidemic hemorrhagic fever in Bolivia. I. A preliminary report of the epidemiological and clinical findings in a new epidemic area in South America. Am. J. Trop. Med. Hyg. 13, 620–625 (1964).
Peters, C.J., Zaki, S.R. & Rollin, P.E. Viral hemorrhagic fevers, in Atlas of Infectious Diseases Vol. 3 (ed Feteky, R.) (Churchill Livingstone, Philadelphia, 1997).
Molinas, F.C., de Bracco, M.M. & Maiztegui, J.I. Coagulation studies in Argentine hemorrhagic fever. J. Infect. Dis. 143, 1–6 (1981).
Dennis, L.H., Reisberg, B.E., Crosbie, J., Crozier, D. & Conrad, M.E. The original haemorrhagic fever: yellow fever. Br. J. Haematol. 17, 455–462 (1969).
Scott, S.K. et al. Studies of the coagulation system and blood pressure during experimental Bolivian hemorrhagic fever in rhesus monkeys. Am. J. Trop. Med. Hyg. 27, 1232–1239 (1978).
Molinas, F.C. et al. Alteration of blood coagulation and complement system in neotropical primates infected with Junin virus. J. Med. Virol. 12, 281–292 (1983).
Salas, R. et al. Venezuelan haemorrhagic fever. Lancet 338, 1033–1036 (1991).
Heller, M.V. et al. Early markers of blood coagulation and fibrinolysis activation in Argentine hemorrhagic fever. Thromb. Haemost. 73, 368–373 (1995).
Geisbert, T.W. et al. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 188, 1618–1629 (2003).
Knobloch, J. et al. Clinical obersvations in 42 patients with Lassa fever. Tropenmed. Parasitol. 31, 389–398 (1980).
Lange, J.V. et al. Kinetic study of platelets and fibrinogen in Lassa virus-infected monkeys and early pathologic events in Mopeia virus-infected monkeys. Am. J. Trop. Med. Hyg. 34, 999–1007 (1985).
Rippey, J.J., Schepers, N.J. & Gear, J.H. The pathology of Marburg virus disease. S. Afr. Med. J. 66, 50–54 (1984).
Isaacson, M., Sureua, P., Courtielle, G. & Pattyn, S.R. Clinical aspects of Ebola virus disease at the Ngaliema hospital, Kinshasa, Zaire, 1976, in Ebola Virus Haemorrhagic Fever (ed. Pattyn, S.R.) (Elsevier/North-Holland Biomedical Press, New York, 1978).
Santos, F. et al. Coagulacao intravascular diseminada aguda na febre amarela: dosagem dos factores da coagulacao. Brasilia Med. 9, 9–16 (1973).
Grignani, G. & Maiolo, A. Cytokines and hemostasis. Haematologica 85, 967–972 (2000).
Neumann, F-J. et al. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler. Thromb. Vasc. Biol. 17, 3399–3405 (1997).
Volchkov, V.E. et al. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity. Science 291, 1965–1969 (2001).
Neumann, G., Feldmann, H., Watanabe, S., Lukashevich, I. & Kawaoka, Y. Reverse genetics demonstrates that proteolytic processing of the Ebola virus glycoprotein is not essential for replication in cell culture. J. Virol. 76, 406–410 (2002).
Flick, R., Flick, K., Feldmann, H. & Elgh, F. Reverse genetics for Crimean-Congo hemorrhagic fever virus. J. Virol. 77, 5997–6006 (2003).
Bredenbeek, P.J. et al. A stable full-length yellow fever virus cDNA clone and the role of conserved RNA elements in flavivirus replication. J. Gen. Virol. 84, 1261–1268 (2003).
Rubins, K.H. et al. Systematic analysis of the gene expression program in peripheral blood cells in response to systemic smallpox infection. Proc. Natl. Acad. Sci. USA 101, 15190–15195 (2004).
Emmert-Buck, M.R. et al. Laser capture microdissection. Science 274, 998–1001 (1996).
Bonner, R.F. et al. Laser capture microdissection: molecular analysis of tissue. Science 278, 1481, 1483 (1997).
Alibek, K. & Handelman, S. Biohazard: The Chilling True Story of the Largest Covert Biological Weapons Program in the World. Told From the Inside by the Man Who Ran It (Random House, New York, 1999).
Monath, T.P. Yellow fever: an update. Lancet Infect. Dis. 1, 11–20 (2001).
Maiztegui, J.I. et al. (1998) Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. J. Infect. Dis. 177, 277–283 (1998).
Pittman, P.R. et al. Immunogenicity of an inactivated Rift Valley fever vaccine in humans. Vaccine 18, 181–189 (1999).
Geisbert, T.W. & Jahrling, P.B. Towards a vaccine against Ebola virus. Expert Rev. Vaccines 2, 777–789 (2003).
Fisher-Hoch, S.P. & McCormick, J.B. Lassa fever vaccine. Expert Rev. Vaccines 3, 189–197 (2004).
Fisher-Hoch, S.P., Hutwagner, L., Brown, B. & McCormick, J.B. Effective vaccine for Lassa fever. J. Virol. 74, 6777–6783 (2000).
Sullivan, N.J., Sanchez, A., Rollin, P.E., Yang, Z-Y. & Nabel, G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408, 605–609 (2000).
Sullivan, N.J. et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424, 681–684.
Jones, S. et al. Replicating vectors for vaccine development. VRC Symposium on Viral Hemorrhagic Fevers, Bethesda, MD, October 14–17, 2003.
International Society for Infectious Diseases. Ebola, lab accident death - Russia (Siberia), May 22, 2004. Archive no. 20040522.1377. http://www.promedmail.org (2004).
Huggins, J., Zhang, Z.X. & Bray, M. Antiviral drug therapy of filovirus infections: S-adenosylhomocysteine hydrolase inhibitors inhibit Ebola virus in vitro and in a lethal mouse model. J. Infect. Dis. 179 Suppl 1, S240–S247 (1999).
Huggins, J.W., Zhang, Z.X., Davis, K. & Coulombe, R.A. Inhibition of Ebola virus by S-adenosylhomocysteine hydrolase inhibitors. Antiviral Res. 26, A301 (1995).
Canonico, P.G., Kende, M., Luscri, B.J. & Huggins, J.W. In-vivo activity of antivirals against exotic RNA viral infections. J. Antimicrob. Chemother. 14, 27–41 (1984).
McCormick, J.B. et al. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 314, 20–26 (1986).
Centers for Disease Control. Management of patients with suspected viral hemorrhagic fever. Morb. Mortal. Wkly. Rep. 37 Suppl 3, 1–16 (1988).
Huggins, J.W. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev. Infect. Dis. 11 Suppl 4, S750–S761 (1989).
Fisher-Hoch, S.P. et al. Crimean Congo-haemorrhagic fever treated with oral ribavirin. Lancet 346, 472–475 (1995).
Kilgore, P.E. et al. Treatment of Bolivian hemorrhagic fever with intravenous ribavirin. Clin. Infect. Dis. 24, 718–722 (1997).
Mardani, M., Jahromi, M.K., Naieni, K.H. & Zeinali, M. The efficacy of oral ribavirin in the treatment of crimean-congo hemorrhagic fever in Iran. Clin. Infect. Dis. 36, 1613–1618 (2003).
Ignat'ev, G.M., Strel'tsova, M.A., Agafonov, A.P., Kashentseva, E.A. & Prozorovskii, N.S. Experimental study of possible treatment of Marburg hemorrhagic fever with desferal, ribavirin, and homologous interferon. Vopr. Virusol. 41, 206–209 (1996).
Jahrling, P.B. et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J. Infect. Dis. 179 Suppl 1, S224–S234 (1999).
Morrill, J.C., Jennings, G.B., Cosgriff, T.M., Gibbs, P.H. & Peters, C.J. (1989) Prevention of Rift Valley fever in rhesus monkeys with interferon-α. Rev. Infect. Dis. 11 Suppl 4, S815–S825 (1989).
Morrill, J.C., Czarniecki, C.W. & Peters, C.J. Recombinant human interferon-gamma modulates Rift Valley fever virus infection in the rhesus monkey. J. Interferon Res. 11, 297–304 (1991).
Leifer, E., Gocke, D.J. & Bourne, H. Lassa fever, a new virus disease in man from West Africa, II: report of a laboratory-acquired infection treated with plasma from a person recently recovered from the disease. Am. J. Trop. Med. Hyg. 19, 677–679 (1970).
Emond, R.T., Evans, B., Bowen, E.T. & Lloyd, G. A case of Ebola virus infection. Br. Med. J. 2, 541–544 (1977).
Maiztegui, J.I., Fernandez, N.J. & de Damilano, A.J. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet 2, 1216–1217 (1979).
Enria, D.A., Briggiler, A.M., Fernandez, N.J., Lewis, S.C. & Maiztegui, J.I. Importance of dose of neutralizing antibodies in treatment of Argentine hemorrhagic fever with immune plasma. Lancet 2, 255–256 (1984).
Frame, J.D., Verbrugge, G.P., Gill, R.G. & Pinneo, L. The use of Lassa fever convalescent plasma in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 78, 319–324 (1984).
Mupapa, K. et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. J. Infect. Dis. 179 Suppl 1, S18–S23 (1999).
White, H.A. Lassa fever: a study of 23 hospital cases. Trans. R. Soc. Trop. Med. Hyg. 66, 390–401 (1972).
Clayton, A.J. (1977) Lassa immune serum. Bull. World Health Organ. 55, 435–439 (1977).
Jahrling, P.B., Frame, J.D., Rhoderick, J.B. & Monson, M.H. Endemic Lassa fever in Liberia, IV: selection of optimally effective plasma for treatment by passive immunization. Trans. R. Soc. Trop. Med. Hyg. 79, 380–384 (1985).
Shepherd, A.J., Swanepoel, R. & Leman, P.A. Antibody response in Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 11 Suppl 4, S801–S806 (1989).
Ge, Q. et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. USA 101, 8676–8681 (2004).
Miura, Y. et al. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J. Exp. Med. 193, 651–660 (2001).
Wang, Q., Miyakawa, Y., Fox, N. & Kaushansky, K. Interferon-alpha directly represses megakaryopoiesis by inhibiting thrombopoietin-induced signaling through induction of SOCS-1. Blood 96, 2093–2099 (2000).
Kayagaki, N., Yamaguchi, N., Nakayama, M., Eto, H. & Okumura, K. & Yagita, H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J. Exp. Med. 189, 1451–1460 (1999).
Schnittler, H-J. & Feldmann, H. Marburg and Ebola hemorrhagic fevers: does the primary course of infection depend on the accessibility of organ-specific macrophages? Clin. Infect. Dis. 27, 404–406 (1998).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Geisbert, T., Jahrling, P. Exotic emerging viral diseases: progress and challenges. Nat Med 10 (Suppl 12), S110–S121 (2004). https://doi.org/10.1038/nm1142
Published:
Issue date:
DOI: https://doi.org/10.1038/nm1142
This article is cited by
-
Rational design of universal immunotherapy for TfR1-tropic arenaviruses
Nature Communications (2020)
-
Structural basis for receptor recognition by Lujo virus
Nature Microbiology (2018)
-
T-bet, but not Gata3, overexpression is detrimental in a neurotropic viral infection
Scientific Reports (2017)
-
Post-exposure treatment of Ebola virus disease in guinea pigs using EBOTAb, an ovine antibody-based therapeutic
Scientific Reports (2016)