Abstract
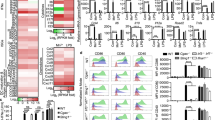
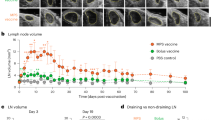
Mast cells (MCs) have recently received recognition as prominent effectors in the regulation of immune cell migration to draining lymph nodes and lymphocyte activation. However, their role in the development of humoral immune responses is not clear. Here, we demonstrate that subcutaneous or nasal administration of small-molecule MC activators with vaccine antigens evokes large increases in antigen-specific serum immunoglobulin G (IgG) responses. These responses were MC dependent and correlated with increased dendritic cell and lymphocyte recruitment to draining lymph nodes. Nasal instillation of these formulations also evoked antigen-specific secretory IgA and provided protection against anthrax lethal toxin challenge in vitro and against vaccinia virus infection in vivo. Collectively, these results define the MC as an integral sensory arm of the adaptive immune system. Moreover, they highlight MC activators as a new class of vaccine adjuvants, capable of inducing protective antigen-specific immune responses through needle-free routes of administration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Echtenacher, B., Mannel, D.N. & Hultner, L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381, 75–77 (1996).
Malaviya, R., Ikeda, T., Ross, E. & Abraham, S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381, 77–80 (1996).
McLachlan, J.B. et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat. Immunol. 4, 1199–1205 (2003).
Jawdat, D.M., Albert, E.J., Rowden, G., Haidl, I.D. & Marshall, J.S. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J. Immunol. 173, 5275–5282 (2004).
Martín-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23, 487–513 (2005).
Gelfand, E.W. Inflammatory mediators in allergic rhinitis. J. Allergy Clin. Immunol. 114, S135–S138 (2004).
Ferry, X., Brehin, S., Kamel, R. & Landry, Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides 23, 1507–1515 (2002).
Aridor, M., Rajmilevich, G., Beaven, M.A. & Sagi-Eisenberg, R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science 262, 1569–1572 (1993).
Paton, W.D. Compound 48/80: a potent histamine liberator. Br. J. Pharmacol. 6, 499–508 (1951).
Krüger, P.-G., Mahata, S.K. & Helle, K.B. Catestatin (chromogranin A344–358) stimulates release of histamine from rat pleural and peritoneal mast cells. Ann. NY Acad. Sci. 971, 349–351 (2002).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Suto, H. et al. Mast cell-associated TNF promotes dendritic cell migration. J. Immunol. 176, 4102–4112 (2006).
Pashine, A., Valiante, N.M. & Ulmer, J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 11, S63–S68 (2005).
Boyaka, P.N. et al. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J. Immunol. 170, 5636–5643 (2003).
Staats, H.F. & Ennis, F.A. Jr. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 162, 6141–6147 (1999).
Yamamoto, S. et al. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 94, 5267–5272 (1997).
Zuercher, A.W., Coffin, S.E., Thurnheer, M.C., Fundova, P. & Cebra, J.J. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J. Immunol. 168, 1796–1803 (2002).
Welle, M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J. Leukoc. Biol. 61, 233–245 (1997).
Befus, A.D., Pearce, F.L., Gauldie, J., Horsewood, P. & Bienenstock, J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J. Immunol. 128, 2475–2480 (1982).
Pearce, F.L. On the heterogeneity of mast cells. Pharmacology 32, 61–71 (1986).
Staats, H.F., Nichols, W.G. & Palker, T.J. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A). J. Immunol. 157, 462–472 (1996).
Palker, T.J. et al. The V3 domain of SIVmac251 gp120 contains a linear neutralizing epitope. Virology 224, 415–426 (1996).
Gaur, R., Gupta, P.K., Banerjea, A.C. & Singh, Y. Effect of nasal immunization with protective antigen of Bacillus anthracis on protective immune response against anthrax toxin. Vaccine 20, 2836–2839 (2002).
Hanna, P. Lethal toxin actions and their consequences. J. Appl. Microbiol. 87, 285–287 (1999).
Fogg, C. et al. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78, 10230–10237 (2004).
Snider, D.P., Marshall, J.S., Perdue, M.H. & Liang, H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J. Immunol. 153, 647–657 (1994).
Brunet, C., Bedard, P.M. & Hebert, J. Analysis of compound 48/80-induced skin histamine release and leukotriene production in chronic urticaria. J. Allergy Clin. Immunol. 82, 398–402 (1988).
Dor, P.J. et al. Induction of late cutaneous reaction by kallikrein injection: comparison with allergic-like late response to compound 48/80. J. Allergy Clin. Immunol. 71, 363–370 (1983).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Dieu, M.C. et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188, 373–386 (1998).
Scandella, E., Men, Y., Gillessen, S., Forster, R. & Groettrup, M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 100, 1354–1361 (2002).
Abraham, S.N. & Arock, M. Mast cells and basophils in innate immunity. Semin. Immunol. 10, 373–381 (1998).
Lindblad, E.B. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 82, 497–505 (2004).
Staats, H.F. et al. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J. Immunol. 167, 5386–5394 (2001).
Acknowledgements
The authors would like to acknowledge S. Kirwan for technical assistance, K. Owzar for assistance with the statistical evaluation of the immunization data for the vaccinia infection study (that is, protection against weight loss), H.-X. Liao (Laboratory of Protein Expression, Duke Human Vaccine Institute) for the recombinant B5R and M. Jenkins (University of Minnesota) for the CD11c-DTR mice used in this study. The following reagents were obtained through the BioDefense and Emerging Infections Research Repository of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health: anthrax protective antigen (PA), recombinant from B. anthracis, NR-140, and anthrax lethal factor recombinant from B. anthracis, NR-570. This work was supported by US National Institutes of Health grants R37-DK50814, RO1-DK077159 and RO1-AI50021.
Author information
Authors and Affiliations
Contributions
J.B.M. and C.P.S. conducted the majority of the experiments; J.P.H. provided discussions and final editing of the manuscript; S.V.P. provided discussion; R.G. assisted with the IgE titers; R.B.-D. provided the NALT micrograph; H.F.S. assisted with the IgG titers and the protection studies and in the design of the study; J.B.M., C.P.S. and S.N.A. designed the study and wrote the manuscript; and the other authors read the paper and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Methods (PDF 758 kb)
Rights and permissions
About this article
Cite this article
McLachlan, J., Shelburne, C., Hart, J. et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med 14, 536–541 (2008). https://doi.org/10.1038/nm1757
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nm1757
This article is cited by
-
New perspectives on the origins and heterogeneity of mast cells
Nature Reviews Immunology (2023)
-
IgE-activated mast cells enhance TLR4-mediated antigen-specific CD4+ T cell responses
Scientific Reports (2021)
-
Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy
npj Vaccines (2020)
-
Mast cell activator compound 48/40 is not an effective adjuvant for UV-attenuated Toxoplasma gondii vaccine
Parasitology Research (2017)
-
The nature of immune responses to urinary tract infections
Nature Reviews Immunology (2015)