Abstract
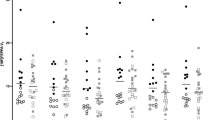
Corticolimbic circuitry has been implicated in generalized social anxiety disorder (gSAD) by several neuroimaging symptom provocation studies. However, there are limited data regarding resting state or treatment effects on regional cerebral metabolic rate of glucose uptake (rCMRglu). Given evidence for anxiolytic effects conferred by tiagabine, a γ-aminobutyric acid (GABA) reuptake inhibitor, the present [18F] fluorodeoxyglucose-positron emission tomography (18FDG-PET) study sought to (1) compare resting rCMRglu between healthy control (HC) and pretreatment gSAD cohorts, (2) examine pre- to post-tiagabine treatment rCMRglu changes in gSAD, and (3) determine rCMRglu predictors of tiagabine treatment response. Fifteen unmedicated individuals with gSAD and ten HCs underwent a baseline (pretreatment) resting-state 18FDG-PET scan. Twelve of the gSAD individuals completed an open, 6-week, flexible dose trial of tiagabine, and underwent a second (posttreatment) resting-state 18FDG-PET scan. Compared to the HC subjects, individuals with gSAD demonstrated less pretreatment rCMRglu within the anterior cingulate cortex and ventral medial prefrontal cortex (vmPFC) at baseline. Following tiagabine treatment, vmPFC rCMRglu increased significantly in the gSAD group. Further, the magnitude of treatment response was inversely correlated with pretreatment rCMRglu within vmPFC. Taken together the present findings converge with neuroimaging findings from studies of social cognition in healthy individuals and symptom provocation in gSAD to support a role for the vmPFC in the pathophysiology of gSAD. Given the pharmacological profile of tiagabine, these findings suggest that its therapeutic effects in gSAD may be mediated by GABAergic modulation within the vmPFC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adolphs R (2003). Cognitive neuroscience of human social behaviour. Nat Rev Neurosci 4: 165–178.
Akirav I, Maroun M (2007). The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast: doi:10.1155/2007/30873.
Amaral DG (2002). The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry 51: 11–17.
Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS (2005). Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 57: 975–981.
Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK (2005). Neurobiological mechanisms of the placebo effect. J Neurosci 25: 10390–10402.
Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C (1994). Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol 269: 219–224.
Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL et al (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875–887.
Crane D (2003). Tiagabine for the treatment of anxiety. Depress Anxiety 18: 51–52.
Dunlop BW, Papp L, Garlow SJ, Weiss PS, Knight BT, Ninan PT (2007). Tiagabine for social anxiety disorder. Hum Psychopharmacol 22: 241–244.
Duvernoy H (1999). The Human Brain. Surface, Three Dimensional Sectional Anatomy with MRI, and Blood Supply. Springer-Wien: New York.
Etkin A, Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488.
Evans KC, Dougherty DD, Pollack MH, Rauch SL (2006). Using neuroimaging to predict treatment response in mood and anxiety disorders. Ann Clin Psychiatry 18: 33–42.
Fink-Jensen A, Suzdak PD, Swedberg MD, Judge ME, Hansen L, Nielsen PG (1992). The gamma-aminobutyric acid (GABA) uptake inhibitor, tiagabine, increases extracellular brain levels of GABA in awake rats. Eur J Pharmacol 220: 197–201.
First MB, Spitzer RL, Gibbon M, Williams JB (1995). Structured Clinical Interview for DSM-IV-TR Axis I Disorders. SCID-I/P. New York State Psychiatric Institute: New York. Research Version.
Friston KJ, Ashburner J, Kiebel SJ, Nichols TE, Penny WD (2007). Statistical Parametric Mapping the Analysis of Functional Brain Images. Academic Press: London.
Frith CD (2007). The social brain? Philos Trans R Soc Lond B Biol Sci 362: 671–678.
Furmark T, Appel L, Michelgard A, Wahlstedt K, Ahs F, Zancan S et al (2005). Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry 58: 132–142.
Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B et al (2002). Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry 59: 425–433.
Geday J, Gjedde A, Boldsen AS, Kupers R (2003). Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage 18: 675–684.
Harlow JM (1868). Recovery from the passage of an iron bar through the head. Pub Mass Med Soc 2: 239–347.
Ipser JC, Kariuki CM, Stein DJ (2008). Pharmacotherapy for social anxiety disorder: a systematic review. Expert Rev Neurother 8: 235–257.
Kent JM, Mathew SJ, Gorman JM (2002). Molecular targets in the treatment of anxiety. Biol Psychiatry 52: 1008–1030.
Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE et al (2006). The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology 31: 2243–2253.
Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK et al (2007). Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry 61: 1081–1089.
Liebowitz MR (1987). Social phobia. Mod Probl Pharmacopsychiatry 22: 141–173.
Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB et al (2004). Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport 15: 2701–2705.
Lydiard RB (2003). The role of GABA in anxiety disorders. J Clin Psychiatry 64: 21–27.
Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S et al (2002). The functional neuroanatomy of the placebo effect. Am J Psychiatry 159: 728–737.
Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL (2005). Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci USA 102: 10706–10711.
Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62: 446–454.
Mitchell JP, Macrae CN, Banaji MR (2006). Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 50: 655–663.
Ninan PT, Papp LA (2004). The Effects of Tiagabine in Patients with Social Anxiety Disorder. Anxiety Disorders Association of America Annual Conference: Miami, FL.
Ongur D, Ferry AT, Price JL (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460: 425–449.
Phan KL, Fitzgerald DA, Cortese BM, Seraji-Bozorgzad N, Tancer ME, Moore GJ (2005). Anterior cingulate neurochemistry in social anxiety disorder: 1H-MRS at 4 Tesla. Neuroreport 16: 183–186.
Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME (2006). Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry 59: 424–429.
Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43: 897–905.
Pollack MH (2001). Comorbidity, neurobiology, and pharmacotherapy of social anxiety disorder. J Clin Psychiatry 62: 24–29.
Quirk GJ, Garcia R, Gonzalez-Lima F (2006). Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60: 337–343.
Rosenthal M (2003). Tiagabine for the treatment of generalized anxiety disorder: a randomized, open-label, clinical trial with paroxetine as a positive control. J Clin Psychiatry 64: 1245–1249.
Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F (2004). Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex 14: 1100–1109.
Schwartz TL, Nasra GS, Ashton AK, Kang D, Kumaresan H, Chilton M et al (2007). An open-label study to evaluate switching from an SSRI or SNRI to tiagabine to alleviate antidepressant-induced sexual dysfunction in generalized anxiety disorder. Ann Clin Psychiatry 19: 25–30.
Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F et al (2000). Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem 75: 732–740.
Shamay-Tsoory SG, Aharon-Peretz J (2007). Dissociable prefrontal networks for cognitive and affective theory of mind: A lesion study. Neuropsychologia 45: 3054–3067.
Spielberger CD, Gorusch RL, Lushene RE (1970). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA.
Stein MB (2006). An epidemiological perspective on social anxiety disorder. J Clin Psychiatry 67: 3–8.
Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG (2002). Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 59: 1027–1034.
Straube T, Mentzel HJ, Miltner WH (2005). Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology 52: 163–168.
Talairach J, Tournoux P (1988). Coplanar Stereotaxic Atlas of the Human Brain. Thieme: Stuttgart.
Tillfors M (2004). Why do some individuals develop social phobia? A review with emphasis on the neurobiological influences. Nord J Psychiatry 58: 267–276.
Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B et al (2001). Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry 158: 1220–1226.
Van Ameringen M, Mancini C, Szechtman H, Nahmias C, Oakman JM, Hall GB et al (2004). A PET provocation study of generalized social phobia. Psychiatry Res 132: 13–18.
Acknowledgements
The study was supported by a grant from Cephalon Inc. Dr Evans was supported by the Clinical Investigator Training Program (Harvard/MIT/HST-BIDMC, in collaboration with Pfizer Inc. and Merck & Co Inc.), the American Psychiatric Research Institute for Research and Education/Merck & Co Early Academic Career Research Award and NIH grants MH T32 19126 and EB T32 1632-03. Dr Rauch was supported in part by NIMH R01 grants MH60219, MH074848, and MH070730. We thank the research participants as well as Diana Fischmann, Elizabeth Scannell, and Steve Weise, for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Previously presented in part as a poster at the 2006 American College of Neuropsychopharmacology Annual Meeting.
DISCLOSURES/CONFLICTS OF INTEREST
This study was supported by an investigator-initiated grant from Cephalon. Additional disclosures from the individual authors are listed below.
Karleyton C Evans
Participation in sponsored clinical trials: Cephalon, Cyberonics, Northstar Neuroscience, Pfizer
Unrestricted research fellowship support: Janssen, Merck, Pfizer
Naomi M Simon
Participation in sponsored clinical trials: NIMH, NARSAD, Astra Zeneca, Cephalon, Forest Laboratories, GlaxoSmithKline, Lilly, Pfizer, UCB-Pharma, Sepracor
Advisory Boards and Consulting: Solvay
Darin D Dougherty
Participation in sponsored clinical trials: McNeil, Cyberonics, Forest Laboratories, Lilly, Medtronics, Northstar Neuroscience
Speaker's Bureau for CME and other presentations: Cyberonics, McNeil
Elizabeth A Hoge
Participation in sponsored clinical trials: Astra Zeneca, Cephalon, Forest Laboratories, GlaxoSmithKline, Janssen, Lilly, Pfizer, UCB-Pharma, Sepracor
Unrestricted research fellowship support: Merck, Pfizer
John J Worthington
Participation in sponsored clinical trials: Abbott Laboratories, Alkermes, Aspect Medical Systems, Astra-Zeneca, Bristol-Myers Squibb Company, Cephalon, Eli Lilly, Forest Pharmaceuticals Inc., GlaxoSmithkline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc., Pharmavite, Roche, Sanofi-Aventis, Sepracor, Solvay Pharmaceuticals Inc., UCB Pharma and Wyeth-Ayerst Laboratories
Speaker's Bureau for CME and other presentations: Bristol-Myers Squibb Company, Eli Lilly, Forest Pharmaceuticals Inc., GlaxoSmithKline, Pfizer Inc., Sanofi-Aventis and Wyeth-Ayerst Laboratories
Candice Chow
No financial interests to disclose.
Rebecca E Kaufman
No financial interests to disclose.
Andrea L Gold
Participation in sponsored clinical trials: Cephalon
Alan J Fischman
Participation in sponsored clinical trials: Cephalon, GlaxoSmithKline, McNeil Inc., Shire Pharmaceuticals Group
Mark H Pollack
Advisory Boards and Consulting: AstraZeneca, Brain Cells Inc., Bristol Myers Squibb, Cephalon, Forest Laboratories, GlaxoSmithKline, Janssen, Jazz Pharmaceuticals, Eli Lilly, Medavante, Neurocrine, Neurogen, Novartis, Otsuka Pharmaceuticals, Pfizer, Predix, Roche, Sanofi, Sepracor, Solvay, Tikvah Therapeutics, Transcept Inc., UCB Pharma, Wyeth
Research Grants: Bristol Myers Squibb, Cephalon, Forest Laboratories, GlaxoSmithKline, Janssen, Eli Lilly, NARSAD, NIDA NIMH, Pfizer, Sepracor, UCB Pharma, Wyeth
Speaker's Bureau for CME and other presentations: Bristol Myers Squibb, Forest Laboratories, GlaxoSmithKline, Janssen, Lilly, Pfizer, Solvay, Wyeth
Equity: Medavante, Mensante Corporation
Scott L Rauch
Consulting: Novartis, Neurogen, Sepracor
Participation in sponsored clinical trials: Cephalon, Cyberonics, Medtronic Inc.
Speaker's Bureau for CME and other presentations: Novartis
Unrestricted research support of laboratory fellows: Pfizer
Rights and permissions
About this article
Cite this article
Evans, K., Simon, N., Dougherty, D. et al. A PET Study of Tiagabine Treatment Implicates Ventral Medial Prefrontal Cortex in Generalized Social Anxiety Disorder. Neuropsychopharmacol 34, 390–398 (2009). https://doi.org/10.1038/npp.2008.69
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2008.69
Keywords
This article is cited by
-
Functional connectivity density abnormalities and anxiety in primary insomnia patients
Brain Imaging and Behavior (2021)
-
Abnormal neural responses to emotional stimuli in children with primary monosymptomatic nocturnal enuresis
European Child & Adolescent Psychiatry (2019)
-
The role of mid-insula in the relationship between cardiac interoceptive attention and anxiety: evidence from an fMRI study
Scientific Reports (2018)
-
Neuroimaging Predictors and Mechanisms of Treatment Response in Social Anxiety Disorder: an Overview of the Amygdala
Current Psychiatry Reports (2018)
-
Resting regional brain metabolism in social anxiety disorder and the effect of moclobemide therapy
Metabolic Brain Disease (2018)