Abstract
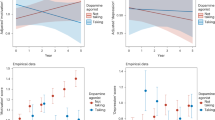
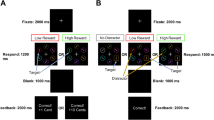
The neurobehavioral underpinnings of pathological gambling are not well understood. Insight might be gained by understanding pharmacological effects on the reward system in patients with Parkinson's disease (PD). Treatment with dopamine agonists (DAs) has been associated with pathological gambling in PD patients. However, how DAs are involved in the development of this form of addiction is unknown. We tested the hypothesis that tonic stimulation of dopamine receptors specifically desensitizes the dopaminergic reward system by preventing decreases in dopaminergic transmission that occurs with negative feedback. Using functional magnetic resonance imaging, we studied PD patients during three sessions of a probabilistic reward task in random order: off medication, after levodopa (LD) treatment, and after an equivalent dose of DA (pramipexole). For each trial, a reward prediction error value was computed using outcome, stake, and probability. Pramipexole specifically changed activity of the orbitofrontal cortex (OFC) in two ways that were both associated with increased risk taking in an out-of-magnet task. Outcome-induced activations were generally higher with pramipexole compared with LD or off medication. In addition, only pramipexole greatly diminished trial-by-trial correlation with reward prediction error values. Further analysis yielded that this resulted mainly from impaired deactivation in trials with negative errors in reward prediction. We propose that DAs prevent pauses in dopamine transmission and thereby impair the negative reinforcing effect of losing. Our findings raise the question of whether pathological gambling may in part stem from an impaired capacity of the OFC to guide behavior when facing negative consequences.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmed SH, Kenny PJ, Koob GF, Markou A (2002). Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci 5: 625–626.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association: Washington, DC; 1994.
Bayer HM, Lau B, Glimcher PW (2007). Statistics of midbrain dopamine neuron spike trains in the awake primate. J Neurophysiol 98: 1428–1439.
Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P (2001). Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30: 619–639.
Cilia R, Siri C, Marotta G, Isaias IU, De Gaspari D, Canesi M et al (2008). Functional abnormalities underlying pathological gambling in Parkinson disease. Arch Neurol 65: 1604–1611.
Cools R (2006). Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev 30: 1–23.
Cools R, Altamirano L, D’Esposito M (2006). Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia 44: 1663–1673.
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001). Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 11: 1136–1143.
Driver-Dunckley E, Samanta J, Stacy M (2003). Pathological gambling associated with dopamine agonist therapy in Parkinson's disease. Neurology 61: 422–423.
Elliott R, Newman JL, Longe OA, Deakin JF (2003). Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci 23: 303–307.
Frank MJ, Samanta J, Moustafa AA, Sherman SJ (2007). Hold your horses: impulsivity, deep brain stimulation, and medication in Parkinsonism. Science 318: 1309–1312.
Frank MJ, Seeberger LC, O’Reilly R C (2004). By carrot or by stick: cognitive reinforcement learning in Parkinsonism. Science 306: 1940–1943.
Friston KJ, Frith CD, Turner R, Frackowiak RS (1995). Characterizing evoked hemodynamics with fMRI. Neuroimage 2: 157–165.
Galpern WR, Stacy M (2007). Management of impulse control disorders in Parkinson's disease. Curr Treat Options Neurol 9: 189–197.
Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ et al (2000). Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157: 1789–1798.
Genovese CR, Lazar NA, Nichols T (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878.
Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F et al (2007). Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend 87: 233–240.
Grigson PS, Twining RC (2002). Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci 116: 321–333.
Hamidovic A, Kang UJ, de Wit H (2008). Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol 28: 45–51.
Hollerman JR, Tremblay L, Schultz W (1998). Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol 80: 947–963.
Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12: 3683–3687.
Knutson B, Westdorp A, Kaiser E, Hommer D (2000). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12: 20–27.
Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI (2009). Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 12: 535–540.
Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL et al (2002). Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). J Exp Psychol Appl 8: 75–84.
O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ (2003). Temporal difference models and reward-related learning in the human brain. Neuron 38: 329–337.
Potenza MN (2008). Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci 363: 3181–3189.
Pontone G, Williams JR, Bassett SS, Marsh L (2006). Clinical features associated with impulse control disorders in Parkinson disease. Neurology 67: 1258–1261.
Ragonese P, Salemi G, Morgante L, Aridon P, Epifanio A, Buffa D et al (2003). A case-control study on cigarette, alcohol, and coffee consumption preceding Parkinson's disease. Neuroepidemiology 22: 297–304.
Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C (2005). Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci 8: 147–148.
Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ et al (2007). Ageing and early-stage Parkinson's disease affect separable neural mechanisms of mesolimbic reward processing. Brain 130: 2412–2424.
Schultz W (2002). Getting formal with dopamine and reward. Neuron 36: 241–263.
Seedat S, Kesler S, Niehaus DJ, Stein DJ (2000). Pathological gambling behavior: emergence secondary to treatment of Parkinson's disease with dopaminergic agents. Depress Anxiety 11: 185–186.
Seeman P (2007). Anti-Parkinson therapeutic potencies correlate with their affinities at dopamine D2(High) receptors. Synapse 61: 1013–1018.
Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA (2004). Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain 127: 851–859.
Steeves TDL, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, van Eimeren T et al (2009). Increased ventral striatal dopamine release in Parkinson's disease patients with pathological gambling: a [11C] raclopride PET study. Brain 132: 1376–1385.
Sutton RS, Barto AG (1998). Reinforcement Learning: An Introduction. MIT Press: Cambridge, MA; 1998.
Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW (2000). Probabilistic learning and reversal deficits in patients with Parkinson's disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia 38: 596–612.
Tomer R, Aharon-Peretz J (2004). Novelty seeking and harm avoidance in Parkinson's disease: effects of asymmetric dopamine deficiency. J Neurol Neurosurg Psychiatry 75: 972–975.
Tremblay L, Schultz W (1999). Relative reward preference in primate orbitofrontal cortex. Nature 398: 704–708.
Valentin VV, Dickinson A, O’Doherty JP (2007). Determining the neural substrates of goal-directed learning in the human brain. J Neurosci 27: 4019–4026.
Volkow ND, Fowler JS, Wang GJ (2004). The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology 47 (Suppl 1): 3–13.
Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F (2009). Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 56 (Suppl 1): 3–8.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R et al (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386: 830–833.
Voon V, Hassan K, Zurowski M, Duff-Canning S, de Souza M, Fox S et al (2006). Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology 66: 1750–1752.
Waelti P, Dickinson A, Schultz W (2001). Dopamine responses comply with basic assumptions of formal learning theory. Nature 412: 43–48.
Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy MA, Whetteckey J et al (2008), for the DOMINION Study Group. Domapinergic Therapy and Impulse Control Disorders in Parkinson's Diesease: Top Line Results of a Cross-Sectional Study of Over 3,000 Patients. 12th International Congress of Parkinson's Disease and Movement Disorders: Chicago, IL; 2008.
White TL, Lejuez CW, de Wit H (2008). Test-retest characteristics of the Balloon Analogue Risk Task (BART). Exp Clin Psychopharmacol 16: 565–570.
Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C (2006). Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci 26: 9530–9537.
Acknowledgements
We thank the staff of the medical imaging department (especially Adrian Crawley) and Movement Disorders center (especially Rosalind Chuang, MD and Thomas Steeves, MD) of the Toronto Western Hospital for their assistance in carrying out the study. This work was partially supported by a grant from the Canadian Institutes of Health Research (MOP-64423 to APS) and Safra Foundation. APS is supported by the Canadian Institutes of Health Research New Investigator Research Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
van Eimeren, T., Ballanger, B., Pellecchia, G. et al. Dopamine Agonists Diminish Value Sensitivity of the Orbitofrontal Cortex: A Trigger for Pathological Gambling in Parkinson's Disease?. Neuropsychopharmacol 34, 2758–2766 (2009). https://doi.org/10.1038/npp.2009.124
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2009.124
Keywords
This article is cited by
-
Dopamine regulates decision thresholds in human reinforcement learning in males
Nature Communications (2023)
-
Impulsive-compulsive behaviour in early Parkinson’s disease is determined by apathy and dopamine receptor D3 polymorphism
npj Parkinson's Disease (2023)
-
Inhibitory framing in hypersexual patients with Parkinson’s disease. An fMRI pilot study
Experimental Brain Research (2022)
-
Medial orbitofrontal cortex dopamine D1/D2 receptors differentially modulate distinct forms of probabilistic decision-making
Neuropsychopharmacology (2021)
-
Unlucky punches: the vulnerability-stress model for the development of impulse control disorders in Parkinson’s disease
npj Parkinson's Disease (2021)