Abstract
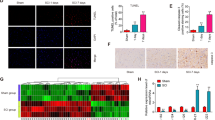
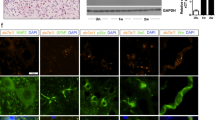
Spinal cord injury (SCI) is a major cause of disability, its clinical outcome depending mostly on the extent of damage in which proapoptotic cytokines have a crucial function. In particular, the inducers of apoptosis belonging to TNF receptor superfamily and their respective ligands are upregulated after SCI. In this study, the function of the proapoptotic cytokine tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in SCI-induced damage was investigated in the mouse. SCI resulted in severe trauma, characterized by prominent inflammation-related damage and apoptosis. Immunostaining for TRAIL and its receptor DR5 was found in the white and gray matter of the perilesional area, as also confirmed by western blotting experiments. Immunoneutralization of TRAIL resulted in improved functional recovery, reduced apoptotic cell number, modulation of molecules involved in the inflammatory response (FasL, TNF-α, IL-1β, and MPO), and the corresponding signaling (caspase-8 and -3 activation, JNK phosphorylation, Bax, and Bcl-2 expression). As glucocorticoid-induced TNF receptor superfamily-related protein (GITR) activated by its ligand (GITRL) contributes to SCI-related inflammation, interactions between TRAIL and GITRL were investigated. SCI was associated with upregulated GITR and GITRL expression, a phenomenon prevented by anti-TRAIL treatment. Moreover, the expression of both TRAIL and DR5 was reduced in tissues from mice lacking the GITR gene (GITR−/−) in comparison with wild-type mice suggesting that TRAIL- and GITRL-activated pathways synergise in the development of SCI-related inflammatory damage. Characterization of new targets within such molecular systems may constitute a platform for innovative treatment of SCI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
28 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41386-023-01768-0
References
Almasan A, Ashkenazi A (2003). Apo2L/TRAIL: apoptosis signalling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev 14: 337–348.
Aktas O, Schulze-Topphoff U, Zipp F (2007). The role of TRAIL/TRAIL receptors in central nervous system pathology. Front Biosci 12: 2912–2921.
Ashkenazi A, Dixit VM (1998). Death receptors: signalling and modulation. Science 281: 1305–1308.
Basso DM, Beattie MS, Bresnahan JC (1995). A sensitive and reliable locomotor ratting scale for open field testing in rats. J Neurotrauma 12: 1–21.
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
Cantarella G, Lempereur L, D’Alcamo MA, Risuglia N, Cardile V, Pennisi G et al (2007). Trail interacts redundantly with nitric oxide in rat astrocytes: potential contribution to neurodegenerative processes. J Neuroimmunol 182: 41–47.
Cantarella G, Uberti D, Carsana T, Lombardo G, Bernardini R, Memo M (2003). Neutralization of TRAIL death pathway protects human neuronal cell line from beta-amyloid toxicity. Cell Death Differ 10: 134–141.
Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L (1998). Acute inflammatory response in spinal cord following impact injury. Exp Neurol 151: 77–88.
Chatham WW, Swaim R, Frohsin Jr H, Heck LW, Miller EJ, Blackburn Jr WD (1993). Degradation of human articular cartilage by neutrophils in synovial fluid. Arthritis Rheum 36: 51–58.
Corazza N, Jakob S, Schaer C, Frese S, Keogh A, Stroka D et al (2006). TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest 116: 2493–2499.
Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C et al (2004). Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med 10: 389–395.
DeVivo MJ, Rutt RD, Stover SL, Fine PR (1987). Employment after spinal cord injury. Arch Phys Med Rehabil 68: 494–498.
Dörr J, Bechmann I, Waiczies S, Aktas O, Walczak H, Krammer PH et al (2002). Lack of tumor necrosis factor-related apoptosis-inducing ligand but presence of its receptors in the human brain. J Neurosci 22: RC209.
Ekshyyan O, Aw TY (2004). Apoptosis in acute and chronic neurological disorders. Front Biosci 9: 1567–1576.
Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD et al (2006). The cellular inflammatory response in human spinal cords after injury. Brain 129: 3249–3269.
Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muià C, Esposito E et al (2008). TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock 29: 32–41.
Genovese T, Mazzon E, Di Paola R, Cannavo G, Muia C, Bramanti P et al (2005). Role of endogenous ligands for the peroxisome proliferators activated receptors alpha in the secondary damage in experimental spinal cord trauma. Exp Neurol 194: 267–278.
Harrington JF, Messier AA, Levine A, Szmydynger-Chodobska J, Chodobski A (2005). Shedding of tumor necrosis factor type 1 receptor after experimental spinal cord injury. J Neurotrauma 22: 919–928.
Hausmann ON (2003). Post-traumatic inflammation following spinal cord injury. Spinal Cord 41: 369–378.
Jaganathan J, Petit JH, Lazio BE, Singh SK, Chin LS (2002). Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in established and primary glioma cell lines. Neurosurg Focus 13: ecp1.
Joshi M, Fehlings MG (2002a). Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma 19: 175–190.
Joshi M, Fehlings MG (2002b). Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 2. Quantitative neuroanatomical assessment and analysis of the relationships between axonal tracts, residual tissue, and locomotor recovery. J Neurotrauma 19: 191–203.
Jurewicz A, Matysiak M, Andrzejak S, Selmaj K (2006). TRAIL-induced death of human adult oligodendrocytes is mediated by JNK pathway. Glia 53: 158–166.
Krausz LT, Bianchini R, Ronchetti S, Fettucciari K, Nocentini G, Riccardi C (2007). GITR-GITRL system, a novel player in shock and inflammation. ScientificWorldJournal 7: 533–566.
Lee YB, Yune TY, Baik SY, Shin YH, Du S, Rhim H et al (2000). Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp Neurol 166: 190–195.
Li GL, Farooque M, Olsson Y (2000). Changes of Fas and Fas ligand immunoreactivity after compression trauma to rat spinal cord. Acta Neuropathol 100: 75–81.
Liu D, Xu GY, Pan E, McAdoo DJ (1999). Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience 93: 1383–1389.
Lu J, Ashwell KW, Waite P (2000). Advances in secondary spinal cord injury: role of apoptosis. Spine 25: 1859–1866.
Martin-Ventura JL, Munoz-Garcia B, Egido J, Blanco-Colio LM (2007). TRAIL and vascular injury. Front Biosci 12: 3656–3667.
Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, Vogel J et al (1999). CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J Neurosci 19: 3809–3817.
Mullane K (1989). Neutrophil-platelet interactions and post-ischemic myocardial injury. Prog Clin Biol Res 301: 39–51.
Nocentini G, Riccardi C (2009). GITR: a modulator of immune response and inflammation. Adv Exp Med Biol 647: 156–173.
Nocentini G, Cuzzocrea S, Genovese T, Bianchini R, Mazzon E, Ronchetti S et al (2008). Glucocorticoid-induced tumor necrosis factor receptor-related (GITR)-Fc fusion protein inhibits GITR triggering and protects from the inflammatory response after spinal cord injury. Mol Pharmacol 73: 1610–1621.
Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C (2007). GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol 37: 1165–1169.
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997a). An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277: 815–818.
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J et al (1997b). The receptor for the cytotoxic ligand TRAIL. Science 276: 111–113.
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A (1996). Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271: 12687–12690.
Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP (2001). Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol 168: 144–154.
Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S (2004). Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis 15: 415–436.
Razmara M, Hilliard B, Ziarani AK, Murali R, Yellayi S, Ghazanfar M et al (2009). Fn14-TRAIL, a chimeric intercellular signal exchanger, attenuates experimental autoimmune encephalomyelitis. Am J Pathol 174: 460–474.
Rivlin AS, Tator CH (1978). Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol 10: 38–43.
Robertson J, Beaulieu JM, Doroudchi MM, Durham HD, Julien JP, Mushynski WE (2001). Apoptotic death of neurons exhibiting peripherin aggregates is mediated by the proinflammatory cytokine tumor necrosis factor-alpha. J Cell Biol 155: 217–226.
Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C (2007). Glucocorticoid-induced TNFR-related protein lowers the threshold of CD28 costimulation in CD8+ T cells. J Immunol 179: 5916–5926.
Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G et al (2004). GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol 34: 613–622.
Satoh JI, Kuroda Y (2001). Alpha-synuclein expression is up-regulated in NTera2 cells during neuronal differentiation but unaffected by exposure to cytokines and neurotrophic factors. Parkinsonism Relat Disord 8: 7–17.
Shea TB (1994). Technical report. An inexpensive densitometric analysis system using a Macintosh computer and a desktop scanner. Biotechniques 16: 1126–1128.
Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D et al (1997). Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277: 818–821.
Springer JE, Azbill RD, Knapp PE (1999). Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med 5: 943–946.
Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT (1998). Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol 152: 74–87.
Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M et al (1997). Role of neutrophils in spinal cord injury in the rat. Neuroscience 79: 1177–1182.
Tator CH (1995). Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol 5: 407–413.
Tator CH, Koyanagi I (1997). Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg 86: 483–492.
Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP et al (2003). Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA 100: 15059–15064.
Uberti D, Cantarella G, Facchetti F, Cafici A, Grasso G, Bernardini R et al (2004). TRAIL is expressed in the brain cells of Alzheimer's disease patients. Neuroreport 15: 579–581.
Uberti D, Ferrari-Toninelli G, Bonini SA, Sarnico I, Benarese M, Pizzi M et al (2007). Blockade of the tumor necrosis factor-related apoptosis inducing ligand death receptor DR5 prevents beta-amyloid neurotoxicity. Neuropsychopharmacology 32: 872–880.
Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N et al (1997). TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 16: 5386–5397.
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK et al (1995). Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3: 673–682.
Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY (1998). Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats. Brain Res Mol Brain Res 59: 135–142.
Yu WR, Liu T, Fehlings TK, Fehlings MG (2009). Involvement of mitochondrial signaling pathways in the mechanism of Fas-mediated apoptosis after spinal cord injury. Eur J Neurosci 29: 114–131.
Zurita M, Vaquero J, Zurita I (2001). Presence and significance of CD-95 (Fas/APO1) expression after spinal cord injury. J Neurosurg 94: 257–264.
Acknowledgements
We are indebted to Dr Vincenzo Guardabasso, Statistical Services, Policlinico ‘G. Rodolico’, Catania, for his skilful and authorable advice. This work has been supported by a PRIN grant from MIUR, Italian Ministry for Research; PRA grant from the University of Catania; the PhD program in Preclinical and Clinical Pharmacology, University of Catania School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cantarella, G., Di Benedetto, G., Scollo, M. et al. Neutralization of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Reduces Spinal Cord Injury Damage in Mice. Neuropsychopharmacol 35, 1302–1314 (2010). https://doi.org/10.1038/npp.2009.234
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2009.234
Keywords
This article is cited by
-
Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration
Journal of Neuroinflammation (2021)
-
Targeting the miRNA-155/TNFSF10 network restrains inflammatory response in the retina in a mouse model of Alzheimer’s disease
Cell Death & Disease (2021)
-
The immune system on the TRAIL of Alzheimer’s disease
Journal of Neuroinflammation (2020)
-
Beneficial effects of curtailing immune susceptibility in an Alzheimer’s disease model
Journal of Neuroinflammation (2019)
-
Ischemic tolerance modulates TRAIL expression and its receptors and generates a neuroprotected phenotype
Cell Death & Disease (2014)