Abstract
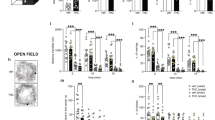
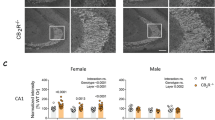
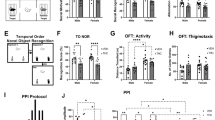
It is firmly established that the hippocampus, a brain region implicated in spatial learning, episodic memory, and consolidation, contains a high concentration of CB1 receptors. Moreover, systemic and intrahippocampal administration of cannabinoid agonists have been shown to impair hippocampal-dependent memory tasks. However, the degree to which CB1 receptors in the hippocampus play a specific functional role in the memory disruptive effects of marijuana or its primary psychoactive constituent Δ9-tetrahydrocannabinol (Δ9-THC) is unknown. This study was designed to determine whether hippocampal CB1 receptors play a functional role in the memory disruptive effects of systemically administered cannabinoids, using the radial arm maze, a well characterized rodent model of working memory. Male Sprague–Dawley rats were implanted with bilateral cannulae aimed at the CA1 region of the dorsal hippocampus. The CB1 receptor antagonist, rimonabant, was delivered into the hippocampus before to a systemic injection of either Δ9-THC or the potent cannabinoid analog, CP-55,940. Strikingly, intrahippocampal administration of rimonabant completely attenuated the memory disruptive effects of both cannabinoids in the radial arm maze task, but did not affect other pharmacological properties of cannabinoids, as assessed in the tetrad assay (that is, hypomotility, analgesia, catalepsy, and hypothermia). Infusions of rimonabant just dorsal or ventral to the hippocampus did not prevent Δ9-THC-induced memory impairment, indicating that its effects on mnemonic function were regionally selective. These findings provide compelling evidence in support of the view that hippocampal CB1 receptors play a necessary role in the memory disruptive effects of marijuana.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brodkin J, Moerschbaecher JM (1997). SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther 282: 1526–1532.
Compton D, Aceto M, Lowe J, Martin B (1996). In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): Inhibition of Δ9-tetrahdrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther 277: 586–594.
Compton DR, Johnson MR, Melvin LS, Martin BR (1992). Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther 260: 201–209.
D'Amour FE, Smith DL (1941). A method for determining loss of pain sensation. J Pharm Exp Ther 72: 74–79.
Deadwyler SA, Goonawardena AV, Hampson RE (2007). Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav Pharmacol 18: 571–580.
Egashira N, Mishima K, Iwasaki K, Fujiwara M (2002). Intracerebral microinjections of delta 9-tetrahydrocannabinol: search for the impairment of spatial memory in the eight-arm radial maze in rats. Brain Res 952: 239–245.
Ferbinteanu J, McDonald RJ (2001). Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus 11: 187–200.
Ferbinteanu J, Ray C, McDonald RJ (2003). Both dorsal and ventral hippocampus contribute to spatial learning in Long-Evans rats. Neurosci Lett 345: 131–135.
Ferrari F, Ottani A, Vivoli R, Giuliani D (1999). Learning impairment produced in rats by the cannabinoid agonist HU 210 in a water-maze task. Pharmacol Biochem Behav 64: 555–561.
Hajos N, Katona I, Naiem S, MacKie K, Ledent C, Mody I et al (2000). Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci 12: 3239–3249.
Hampson RE, Deadwyler SA (1999). Cannabinoids, hippocampal function and memory. Life Sci 65: 715–723.
Hampson RE, Deadwyler SA (2000). Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci 20: 8932–8942.
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: 563–583.
Heyser CJ, Hampson RE, Deadwyler SA (1993). Effects of Δ9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther 264: 294–307.
Hoffman AF, Lupica CR (2000). Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci 20: 2470–2479.
Hoffman AF, Oz M, Caulder T, Lupica CR (2003). Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci 23: 4815–4820.
Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR (2007). Opposing actions of chronic {Delta}9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem 14: 63–74.
Iversen L (2003). Cannabis and the brain. Brain 126: 1252–1270.
Jarbe TU, McMillan DE (1980). delta 9-THC as a discriminative stimulus in rats and pigeons: generalization to THC metabolites and SP-111. Psychopharmacology (Berl) 71: 281–289.
Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE (2007). Monitoring the Future national survey results on drug use, 1975-2006. Volume II: college students and adults ages 19-45 (NIH Publication No. 07-6206). National Institute on Drug Abuse, National Institute on Drug Abuse.
Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K et al (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19: 4544–4558.
Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D et al (2006). Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci 26: 5628–5637.
Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M et al (2006). The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26: 2991–3001.
Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F et al (1999). Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283: 401–404.
Lichtman AH, Dimen KR, Martin BR (1995). Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology 119: 282–290.
Lichtman AH, Martin BR (1996). Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl) 126: 125–131.
Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR (1988). Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther 247: 1046–1051.
Mallet PE, Beninger RJ (1998). The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta-9-tetrahydrocannabinol or anandamide. Psychopharmacology 140: 11–19.
Marsicano G, Lutz B (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11: 4213–4225.
Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ (2004). A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci 7: 585–586.
Matsuda LA, Bonner TI, Lolait SJ (1993). Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol 327: 535–550.
Mishima K, Egashira N, Hirosawa N, Fujii M, Matsumoto Y, Iwasaki K et al (2001). Characteristics of learning and memory impairment induced by delta9-tetrahydrocannabinol in rats. Jpn J Pharmacol 87: 297–308.
Misner DL, Sullivan JM (1999). Mechanism of cannabinoid effects on long-term potential and depression in hippocampal CA1 neurons. J Neurosci 19: 6795–6805.
Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G et al (2007). Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol 5: e269.
Nakamura EM, da Silva EA, Concilio GV, Wilkinson DA, Masur J (1991). Reversible effects of acute and long-term administration of Δ9-tetrahydrocannabinol (THC) on memory in the rat. Drug Alc Depend 28: 167–175.
Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M (2002). Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci 22: 3864–3872.
Olton DS (1987). The radial arm maze as a tool in behavioral pharmacology. Physiol Behav 40: 793–797.
Paxinos G, Watson C (2007). The Rat Brain in Stereotaxic Coordinates, 6th edn. Elsevier: Elsevier, Academic Press, San Diego, CA.
Pertwee RG, Wickens AP (1991). Enhancement by chlordiazepoxide of catalepsy induced in rats by intravenous or intrapallidal injections of enantiomeric cannabinoids. Neuropharmacol 30: 237–244.
Ranganathan M, D'Souza DC (2006). The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berlin) 188: 425–444.
Riedel G, Davies SN (2005). Cannabinoid function in learning, memory and plasticity. Handbook Exp Pharmacol 168: 445–477.
Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C et al (1994). SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350: 240–244.
Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G (2006). Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci 9: 1526–1533.
Shen M, Piser TM, Seybold VS, Thayer SA (1996). Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci 16: 4322–4334.
Silva de Melo LC, Cruz AP, Rios Valentim Jr SJ, Marinho AR, Mendonca JB, Nakamura-Palacios EM (2005). Delta(9)-THC administered into the medial prefrontal cortex disrupts the spatial working memory. Psychopharmacology (Berl) 183: 54–64.
Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR (1994). The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther 270: 219–227.
Suenaga T, Ichitani Y (2008). Effects of hippocampal administration of a cannabinoid receptor agonist WIN 55,212-2 on spontaneous object and place recognition in rats. Behav Brain Res 190: 248–252.
Suenaga T, Kaku M, Ichitani Y (2008). Effects of intrahippocampal cannabinoid receptor agonist and antagonist on radial maze and T-maze delayed alternation performance in rats. Pharmacol Biochem Behav 91: 91–96.
Varvel SA, Anum E, Niyuhire F, Wise LE, Lichtman AH (2004a). Delta(9)-THC-induced cognitive deficits in mice are reversed by the GABA(A) antagonist bicuculline. Psychopharmacology (Berl) 178: 317–327.
Varvel SA, Cichewicz DL, Lichtman AH (2004b). Interactions Between Cannabinoids and Opioids. In: Wenger T (ed). Recent Advances on Pharmacology and Physiology of Cannabinoids. Research Signpost: Kerala, India. pp 157–182.
Varvel SA, Hamm RJ, Martin BR, Lichtman AH (2001). Differential effects of delta9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 157: 142–150.
Varvel SA, Lichtman AH (2002). Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther 301: 915–924.
Wegener N, Kuhnert S, Thuns A, Roese R, Koch M (2008). Effects of acute systemic and intra-cerebral stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats. Psychopharmacology (Berl) 198: 375–385.
Yim TT, Hong NS, Ejaredar M, McKenna JE, McDonald RJ (2008). Post-training CB1 cannabinoid receptor agonist activation disrupts long-term consolidation of spatial memories in the hippocampus. Neuroscience 151: 929–936.
Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI (1999). Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA 96: 5780–5785.
Acknowledgements
We thank Dr. Carl Lupica for his insightful comments and suggestions on an earlier version of this paper. This research was supported by the National Institute on Drug Abuse (R01DA 015683, R01DA003672, and T23DA07027).
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
None of the authors report any conflicts of interest with the work presented in this paper. This research has been supported solely by the National Institutes of Health (NIH). AHL declares that over the past 3 years that he has received compensation from Pfizer, Ironwood Pharmaceuticals, and Allergan. In addition, AHL has received funding from Pfizer and Ironwood for contracts unrelated to the research presented in this paper. AJP declares full-time employment at Abbott, since completing his postdoctoral fellowship and contributions to this paper. LEW declares no financial support or compensation has been received from any individual or corporate entity over the past 3 years.
Rights and permissions
About this article
Cite this article
Wise, L., Thorpe, A. & Lichtman, A. Hippocampal CB1 Receptors Mediate the Memory Impairing Effects of Δ9-Tetrahydrocannabinol. Neuropsychopharmacol 34, 2072–2080 (2009). https://doi.org/10.1038/npp.2009.31
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2009.31
Keywords
This article is cited by
-
The Impact of Cannabis Use on Cognition in People with HIV: Evidence of Function-Dependent Effects and Mechanisms from Clinical and Preclinical Studies
Current HIV/AIDS Reports (2024)
-
The neural and molecular basis of working memory function in psychosis: a multimodal PET-fMRI study
Molecular Psychiatry (2021)
-
Astrocytic Mechanisms Involving Kynurenic Acid Control Δ9-Tetrahydrocannabinol-Induced Increases in Glutamate Release in Brain Reward-Processing Areas
Molecular Neurobiology (2019)
-
Hippocampal Protein Kinase C Signaling Mediates the Short-Term Memory Impairment Induced by Delta9-Tetrahydrocannabinol
Neuropsychopharmacology (2018)
-
Cannabis-related hippocampal volumetric abnormalities specific to subregions in dependent users
Psychopharmacology (2017)