Abstract
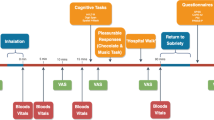
Worldwide cannabis dependence is increasing, as is the concentration of Δ9-tetrahydrocannabinol (THC) in street cannabis. At the same time, the concentration of the second most abundant cannabinoid in street cannabis, cannabidiol (CBD), is decreasing. These two cannabinoids have opposing effects both pharmacologically and behaviorally when administered in the laboratory. No research has yet examined how the ratio of these constituents impacts on the appetitive/reinforcing effects of cannabis in humans. A total of 94 cannabis users were tested 7 days apart, once while non-intoxicated and once while acutely under the influence of their own chosen smoked cannabis on dependence-related measures. Using an unprecedented methodology, a sample of cannabis (as well as saliva) was collected from each user and analyzed for levels of cannabinoids. On the basis of CBD : THC ratios in the cannabis, individuals from the top and bottom tertiles were directly compared on indices of the reinforcing effects of drugs, explicit liking, and implicit attentional bias to drug stimuli. When intoxicated, smokers of high CBD : THC strains showed reduced attentional bias to drug and food stimuli compared with smokers of low CBD : THC. Those smoking higher CBD : THC strains also showed lower self-rated liking of cannabis stimuli on both test days. Our findings suggest that CBD has potential as a treatment for cannabis dependence. The acute modulation of the incentive salience of drug cues by CBD may possibly generalize to a treatment for other addictive disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Berridge KC, Robinson TE, Aldridge JW (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9: 65–73.
Bitencourt RM, Pamplona FA, Takahashi RN (2008). Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol 18: 849–859.
Chait LD, Zacny JP (1992). Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 107: 255–262.
Chopra IC, Chopra RN (1957). The use of cannabis drugs in India. Bull Narc 9: 4–29.
Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A (2007). Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370: 1706–1713.
Cooper ZD, Haney M (2009). Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psychiatry 21: 104–112.
Curran HV, Brignell C, Fletcher S, Middleton P, Henry J (2002). Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 164: 61–70.
D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT et al (2004). The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29: 1558–1572.
D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T et al (2008). Blunted psychotomimetic and amnestic effects of Delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 33: 2505–2516.
Duka T, Townshend JM (2004). The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology (Berl) 176: 353–361.
EMCDDA (2006). Annual Report 2006: The State of the Drugs Problem in Europe. European Monitoring Centre for Drugs and Drug Addiction: Lisbon. Pamphlet.
Fadardi JS, Cox WM (2009). Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depend 101: 137–145.
Field M, Eastwood B, Bradley BP, Mogg K (2006). Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend 85: 75–82.
Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W et al (1995). The severity of dependence scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 90: 607–614.
Guimares FS, Chiaretti TM, Graeff FG (1990). Antianxiety effects of cannabidiol in the elevated plus-maze. Psychopharmacology 100: 558–559.
Hardwick S, King LA (2008). Home Office Cannabis Potency Study. Home Office Scientific Development Branch: St Albans. Report.
Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA (2009). Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend 102: 35–40.
Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G et al (2007). Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry 61: 1281–1289.
Kelly TH, Foltin RW, Emurian CS, Fischman MW (1997). Are choice and self-administration of marijuana related to delta 9-THC content? Exp Clin Psychopharmacol 5: 74–82.
Kirkham TC (2009). Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry 21: 163–171.
Mahler SV, Smith KS, Berridge KC (2007). Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 32: 2267–2278.
Marissen MA, Franken IH, Waters AJ, Blanken P, van den BW, Hendriks VM (2006). Attentional bias predicts heroin relapse following treatment. Addiction 101: 1306–1312.
Morgan CJA, Rees H, Curran HV (2008). Attentional bias to incentive stimuli in frequent ketamine users. Pschol Med 38: 1331–1340.
Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R (2004). Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology 175: 360–366.
Pertwee RG (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153: 199–215.
Ren Y, Whittard J, Higuera-Matas A, Morris CV, Hurd YL (2009). Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci 29: 14764–14769.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291.
Robinson TE, Berridge KC (2001). Incentive-sensitization and addiction. Addiction 96: 103–114.
Robinson TE, Berridge KC (2003). Addiction. Annu Rev Psychol 54: 25–53.
Robinson TE, Berridge KC (2008). Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363: 3137–3146.
Rodriguez dF, Navarro M, Gomez R, Escuredo L, Nava F, Fu J . et al (2001). An anorexic lipid mediator regulated by feeding. Nature 414: 209–212.
Solowij N, Hall W, Lee N (1992). Recreational MDMA use in Sydney: a profile of ‘Ecstasy’ users and their experience with the drug. Br J Addict 87: 1161–1172.
Taylor D (2009). Withdrawal of Rimonabant—walking the tightrope of 21st century pharmaceutical regulation? Curr Drug Saf 4: 2–4.
Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, Wiley JL (2008). Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend 94: 191–198.
Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH (2003). Attentional bias predicts outcome in smoking cessation. Health Psychol 22: 378–387.
Wechsler D (2001). Wechsler Test of Adult Reading. Psychological Corportation: San Antonio, TX.
Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimaraes FS (2006). Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res 39: 421–429.
Acknowledgements
This work was supported by a grant to HVC and CJAM from the Medical Research Council (UK). We thank the Home Office and the Forensic Science Service for their support of the study. We also thank all the participants who donated their time and cannabis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Morgan, C., Freeman, T., Schafer, G. et al. Cannabidiol Attenuates the Appetitive Effects of Δ9-Tetrahydrocannabinol in Humans Smoking Their Chosen Cannabis. Neuropsychopharmacol 35, 1879–1885 (2010). https://doi.org/10.1038/npp.2010.58
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2010.58
Keywords
This article is cited by
-
The acute effects of cannabis, with and without cannabidiol, on attentional bias to cannabis related cues: a randomised, double-blind, placebo-controlled, cross-over study
Psychopharmacology (2024)
-
Effects of cannabidiol on anandamide levels in individuals with cannabis use disorder: findings from a randomised clinical trial for the treatment of cannabis use disorder
Translational Psychiatry (2023)
-
Different Tokes for Different Folks: Use of Cannabis Products Among a Longitudinal Cohort of People with Heroin Dependence
International Journal of Mental Health and Addiction (2023)
-
Biological activity of Cannabis compounds: a modern approach to the therapy of multiple diseases
Phytochemistry Reviews (2022)
-
Cannabidiol Interactions with Medications, Illicit Substances, and Alcohol: a Comprehensive Review
Journal of General Internal Medicine (2021)