Abstract
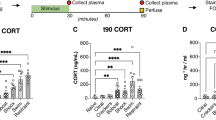
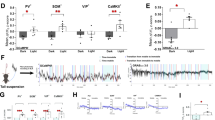
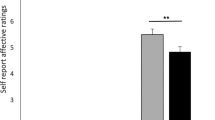
Norepinephrine (NE) is known to play an integral role in the neurobiological response to stress. Exposure to stressful stimuli increases NE levels in brain regions that regulate stress and anxiety, like the basolateral amygdala (BLA). NE is thought to increase excitability in these areas through α- and β-adrenoceptors (ARs), leading to increased anxiety. Surprisingly, recent studies have shown that systemic β3-AR agonist administration decreases anxiety-like behaviors, suggesting that β3-ARs may inhibit excitability in anxiety-related brain regions. Therefore, in this study we integrated electrophysiological and behavioral approaches to test the hypothesis that the anxiolytic effects of β3-AR agonists may be mediated by an increase in BLA GABAergic inhibition. We examined the effect of a selective β3-AR agonist, BRL37344 (BRL), on GABAergic synapses arising from local circuit interneurons and inhibitory synapses originating from a recently described population of cells called lateral paracapsular (LPCS) interneurons. Surprisingly, BRL selectively enhanced LPCS-evoked inhibitory postsynaptic currents (eIPSCs) with no effect on local GABAergic inhibition. BRL also had no effect on glutamatergic synaptic excitation within the BLA. BRL potentiation of LPCS eIPSCs was blocked by the selective β3-AR antagonist, SR59230A, or by intracellular dialysis of Rp-CAMPS (cAMP-dependent protein kinase inhibitor), and this enhancement was not associated with any changes in spontaneous IPSCs or LPCS paired-pulse ratio. BRL also increased the amplitude of unitary LPCS IPSCs (uIPSCs) with no effect on uIPSC failure rate. Finally, bilateral BLA microinjection of BRL reduced anxiety-like behaviors in an open-field assay and the elevated plus-maze. Collectively, these data suggest that β3-AR activation selectively enhances LPCS, but not local, BLA GABAergic synapses, and that increases in LPCS-mediated inhibition may contribute to the anxiolytic profile of β3-AR agonists.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abraham PA, Xing G, Zhang L, Yu EZ, Post R, Gamble EH et al (2008). beta1- and beta2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain Res 1209: 65–73.
Aggleton JP, Burton MJ, Passingham RE (1980). Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res. 190: 347–368.
Arch JR, Ainsworth AT, Cawthorne MA, Piercy V, Sennitt MV, Thody VE et al (1984). Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature 309: 163–165.
Ariwodola OJ, Weiner JL (2004). Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci 24: 10679–10686.
Auger C, Marty A (2000). Quantal currents at single-site central synapses. J Physiol 526: 3–11.
Baker JG (2005). The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 144: 317–322.
Berlau DJ, McGaugh JL (2006). Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem 86: 123–132.
Braga MF, Aroniadou-Anderjaska V, Xie J, Li H (2003). Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci 23: 442–452.
Bremner JD, Krystal JH, Southwick SM, Charney DS (1996). Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 23: 28–38.
Bueno CH, Zangrossi Jr H, Viana MB (2005). The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Braz J Med Biol Res 38: 1697–1701.
Claustre Y, Leonetti M, Santucci V, Bougault I, Desvignes C, Rouquier L et al (2008). Effects of the beta(3)-adrenoceptor (Adrb3) agonist SR58611A (amibegron) on serotonergic and noradrenergic transmission in the rodent: Relevance to its antidepressant/anxiolytic-like profile. Neuroscience 156: 353–364.
Consoli D, Leggio GM, Mazzola C, Micale V, Drago F (2007). Behavioral effects of the beta3 adrenoceptor agonist SR58611A: is it the putative prototype of a new class of antidepressant/anxiolytic drugs? Eur J Pharmacol 573: 139–147.
Daly JW, Padgett W, Creveling CR, Cantacuzene D, Kirk KL (1981). Cyclic AMP-generating systems: regional differences in activation by adrenergic receptors in rat brain. J Neurosci 1: 49–59.
Davis M, Rainnie D, Cassell M (1994). Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci 17: 208–214.
Doheny HC, Lynch CM, Smith TJ, Morrison JJ (2005). Functional coupling of beta3-adrenoceptors and large conductance calcium-activated potassium channels in human uterine myocytes. J Clin Endocrinol Metab 90: 5786–5796.
Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD et al (2005). Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30: 657–668.
Engin E, Treit D (2008). The effects of intra-cerebral drug infusions on animals' unconditioned fear reactions: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 32: 1399–1419.
Fallon JH, Koziell DA, Moore RY (1978). Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol 180: 509–532.
Ferry B, Roozendaal B, McGaugh JL (1999). Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J Neurosci 19: 5119–5123.
File SE, Mabbutt PS, Hitchcott PK (1990). Characterisation of the phenomenon of ‘one-trial tolerance’ to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze. Psychopharmacology 102: 98–101.
File SE, Zangrossi Jr H (1993). ‘One-trial tolerance’ to the anxiolytic actions of benzodiazepines in the elevated plus-maze, or the development of a phobic state? Psychopharmacology 110: 240–244.
Gonzalez LE, Andrews N, File SE (1996). 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res 732: 145–153.
Holmes A, Rodgers RJ (1999). Influence of spatial and temporal manipulations on the anxiolytic efficacy of chlordiazepoxide in mice previously exposed to the elevated plus-maze. Neurosci Biobehav Rev 23: 971–980.
Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV (2008). Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353.
Isoardi NA, Bertotto ME, Martijena ID, Molina VA, Carrer HF (2007). Lack of feedback inhibition on rat basolateral amygdala following stress or withdrawal from sedative-hypnotic drugs. Eur J Neurosci 26: 1036–1044.
Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA (2005). An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol 94: 4491–4501.
Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H (2009). Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology 34: 410–423.
Kapur J, MacDonald RL (1996). Cyclic AMP-dependent protein kinase enhances hippocampal dentate granule cell GABAA receptor current. J Neurophysiol 76: 2626–2634.
Kaneko K, Tamamaki N, Owada H, Kakizaki T, Kume N, Totsuka M et al (2008). Noradrenergic excitation of a subpopulation of GABAergic cells in the basolateral amygdala via both activation of nonselective cationic conductance and suppression of resting K+ conductance: a study using glutamate decarboxylase 67-green fluorescent protein knock-in mice. Neuroscience 157: 781–797.
Kobayashi M, Hamada T, Kogo M, Yanagawa Y, Obata K, Kang Y (2008). Developmental profile of GABAA-mediated synaptic transmission in pyramidal cells of the somatosensory cortex. Eur J Neurosci 28: 849–861.
Kullmann FA, Limberg BJ, Artim DE, Shah M, Downs TR, Contract D et al (2009). Effects of beta3-adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. J Pharmacol Exp Ther 330: 704–717.
Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA (2008). Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology 55: 661–668.
LaLumiere RT, Buen TV, McGaugh JL (2003). Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci 23: 6754–6758.
Leibmann L, Karst H, Joëls M (2009). Effects of corticosterone and the β-agonist isoproterenol on glutamate receptor-mediated synaptic currents in the rat basolateral amygdala. Eur J Neurosci 30: 800–807.
LeDoux J (2003). The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738.
LeDoux JE, Farb CR, Romanski LM (1991). Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci Lett 134: 139–144.
Liu W, Yuen EY, Allen PB, Feng J, Greengard P, Yan Z (2006). Adrenergic modulation of NMDA receptors in prefrontal cortex is differentially regulated by RGS proteins and spinophilin. Proc Natl Acad Sci USA 103: 18338–18343.
Lowell BB, Flier JS (1997). Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annu Rev Med 48: 307–316.
Marowsky A, Yanagawa Y, Obata K, Vogt KE (2005). A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48: 1025–1037.
McCool BA, Chappell A (2007). Strychnine and taurine modulation of amygdala-associated anxiety-like behavior is ‘state’ dependent. Behav Brain Res 178: 70–81.
McGaugh JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28.
Menard J, Treit D (1999). Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev 23: 591–613.
Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S et al (2005). Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 29: 1214–1224.
Oriowo MA, Chapman H, Kirkham DM, Sennitt MV, Ruffolo Jr RR, Cawthorne MA (1996). The selectivity in vitro of the stereoisomers of the beta-3 adrenoceptor agonist BRL 37344. J Pharmacol Exp Ther 277: 22–27.
Ortiz JP, Heinricher MM, Selden NR (2007). Noradrenergic agonist administration into the central nucleus of the amygdala increases the tail-flick latency in lightly anesthetized rats. Neuroscience 148: 737–743.
Paxinos G, Watson C (1997). The Rat Brain in Stereotaxic Coordinates, 4th edn Academic Press: San Diego, CA.
Pesold C, Treit D (1995). The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res 671: 213–221.
Prut L, Belzung C (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463: 3–33.
Qu LL, Guo NN, Li BM (2008). Beta1- and beta2-adrenoceptors in basolateral nucleus of amygdala and their roles in consolidation of fear memory in rats. Hippocampus 18: 1131–1139.
Rodriguez M, Carillon C, Coquerel A, Le Fur G, Ferrara P, Caput D et al (1995). Evidence for the presence of beta 3-adrenergic receptor mRNA in the human brain. Brain Res Mol Brain Res 29: 369–375.
Rozec B, Gauthier C (2006). beta3-adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther 111: 652–673.
Sanders SK, Shekhar A (1995). Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav 52: 701–706.
Selvage DJ, Rivier C (2003). Importance of the paraventricular nucleus of the hypothalamus as a component of a neural pathway between the brain and the testes that modulates testosterone secretion independently of the pituitary. Endocrinology 144: 594–598.
Siggins GR, Roberto M, Nie Z (2005). The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther 107: 80–98.
Silberman Y, Ariwodola OJ, Weiner JL (2009). Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology 56: 886–895.
Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL (2008). Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther 324: 251–260.
Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O et al (2008). Stimulation of the beta3-adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology 33: 574–587.
Stevens CF, Wang Y (1994). Changes in reliability of synaptic function as a mechanism for plasticity. Nature 371: 704–707.
Stevens DR, Kuramasu A, Eriksson KS, Selbach O, Haas HL (2004). Alpha 2-adrenergic receptor-mediated presynaptic inhibition of GABAergic IPSPs in rat histaminergic neurons. Neuropharmacology 46: 1018–1022.
Summers RJ, Papaioannou M, Harris S, Evans BA (1995). Expression of beta 3-adrenoceptor mRNA in rat brain. Br J Pharmacol 116: 2547–2548.
Ursino MG, Vasina V, Raschi E, Crema F, De Ponti F (2009). The beta3-adrenoceptor as a therapeutic target: current perspectives. Pharmacol Res 59: 221–234.
Vrydag W, Michel MC (2007). Tools to study beta3-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 374: 385–398.
Woodruff AR, Sah P (2007). Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci 27: 553–563.
Yurtcu N, Cetin A, Karadas B, Gonca Imir A, Kaya T, Erselcan T et al (2006). Comparison of effects of formoterol and BRL 37344 on isolated term-pregnant rat myometrial strips in vitro. Eur J Pharmacol 530: 263–269.
Acknowledgements
This work was funded by funded by AA 13960, AA 17531, AA 17056, AA 10422 and AA 17039.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Silberman, Y., Ariwodola, O., Chappell, A. et al. Lateral Paracapsular GABAergic Synapses in the Basolateral Amygdala Contribute to the Anxiolytic Effects of β3 Adrenoceptor Activation. Neuropsychopharmacol 35, 1886–1896 (2010). https://doi.org/10.1038/npp.2010.59
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2010.59
Keywords
This article is cited by
-
Lost in translation: no effect of repeated optogenetic cortico-striatal stimulation on compulsivity in rats
Translational Psychiatry (2021)
-
The Role of β1,2-Adrenoceptors in the Amygdala in the Behavior of Rats with Different Levels of Freezing in Conditioned Reflex Fear
Neuroscience and Behavioral Physiology (2019)
-
The BigLEN-GPR171 Peptide Receptor System Within the Basolateral Amygdala Regulates Anxiety-Like Behavior and Contextual Fear Conditioning
Neuropsychopharmacology (2017)
-
Anxiolytic-like effects of α-asarone in a mouse model of chronic pain
Metabolic Brain Disease (2017)
-
Effect of β3 adrenoceptor activation in the basolateral amygdala on ethanol seeking behaviors
Psychopharmacology (2014)