Abstract
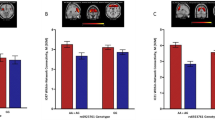
As acute ethanol exposure inhibits N-methyl-D-aspartate glutamate (Glu) receptors, sudden withdrawal from chronic alcohol use may lead to an increased activation of these receptors with excitotoxic effects. In the longer term, brain levels of Glu and its metabolites, such as glutamine (Gln), are likely to be chronically altered by alcohol, possibly providing a measure of overall abnormal Glu–Gln cycling. However, few studies have assessed concentrations of these metabolites in clinical populations of individuals with alcohol use disorders. Glu and Gln levels were compared in groups of 17 healthy controls and in 13 participants with alcohol dependence. Within the alcohol-dependent group, seven participants had current alcohol use disorder (AUD), and six had AUD in remission for at least 1 year (AUD-R). Neurometabolite concentrations were measured with proton magnetic resonance spectroscopy (1H-MRS) in a predominantly gray matter voxel that included the bilateral anterior cingulate gyri. Tissue segmentation provided an assessment of the proportion of gray matter in the 1H-MRS voxel. The Drinker Inventory of Consequences (DrInC) and Form-90 were administered to all participants to quantify alcohol consequences and use. Glu level was lower and Gln level was higher in the AUD and AUD-R groups relative to the control group; creatine, choline, myo-inositol, and total N-acetyl groups, primarily N-acetylaspartate did not differ across groups. These results were not confounded by age, sex, or proportion of gray matter in the 1H-MRS voxel. Neurometabolite concentrations did not differ between AUD and AUD-R groups. Subsequent regressions in the combined clinical group, treating voxel gray matter proportion as a covariate, revealed that total score on the DrInC was positively correlated with Gln but negatively correlated with both Glu and gray matter proportion. Regression analyses, including DrInC scores and smoking variables, identified a marginal independent effect of smoking on Gln. The current findings of higher Gln and lower Glu in the combined AUD and AUD-R groups might indicate a perturbation of the Glu–Gln cycle in alcohol use disorders. The absence of differences in mean Glu and Gln between the AUD and AUD-R groups suggests that altered Glu–Gln metabolism may either predate the onset of abuse or persist during prolonged abstinence.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders: 4th edn. American Psychiatric Association: Washington, DC.
Bisaga A, Popik P (2000). In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-D-aspartate (NMDA) antagonists. Drug and Alcohol Depend. 59: 1–15.
Bustillo JR, Chen H, Gasparovic C, Mullins P, Caprihan A, Qualls C et al (2011). Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 Tesla. Biol Psychiatry 69: 19–27.
Coyle JT, Puttfarcken P (1993). Oxidative stress, glutamate, and neurodegenerative disorders. Science 262: 689–695.
Dahchour A, De Witte P (2003). Effects of acamprosate on excitatory amino acids during multiple ethanol withdrawal periods. Alcohol Clin Exp Res 27: 465–470.
De Witte P (2004). Imbalance between neuroexcitatory and neuroinhibitory amino acids causes craving for ethanol. Addict Behav 29: 1325–1339.
Durazzo TC, Gazdzinski S, Bayns P, Meyerhoff DJ (2004). Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res 28: 1849–1860.
Frye G, Fincher A (2000). Sustained ethanol inhibition of native AMPA receptors on medial septum/diagonal band (MS/DB) neurons. Br J Pharmacol 129: 87–94.
Fein G, Meyerhoff DJ (2000). Ethanol in human brain by magnetic resonance spectroscopy: correlation with blood and breath levels, relaxation, and magnetization transfer. Alcohol Clin Exp Res 24: 1227–1235.
Fein G, Sclafani V, Cardenas VA, Goldman H, Tolou-Shams M, Meyerhoff DJ (2002). Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res 26: 558–564.
Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins P et al (2006). Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55: 1219–1226.
Gass J, Olive M (2008). Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75: 218–265.
Hoffman PL (1995). Glutamate receptors in alcohol withdrawal-induced neurotoxicity. Metab Brain Dis 10: 73–79.
Jagannathan NR, Desai NG, Raghunathan P (1996). Brain metabolite changes in alcoholism: an in vivo proton magnetic resonance spectroscopy (MRS) study. Magn Reson Imaging 14: 553–557.
Lee E, Jang DP, Kim JJ, Kyoon AS, Sangjin P, In-Young K et al (2007). Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport 18: 1511–1514.
Lenth RV (2006–2009). Java applets for power and sample size [computer software]. Retrieved 16 December 2010 from http://www.stat.uiowa.edu/∼rlenth/Power.
Licata SC, Renshaw PF (2010). Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci 1187: 148–171.
Lovinger DM (1993). High ethanol sensitivity of recombinant AMPA-type glutamate receptors expressed in mammalian cells. Neurosci Lett 159: 83–87.
Magistretti PJ, Pellerin L (1999). Astrocytes couple synaptic activity to glucose utilization in the brain. News Physiol Sci. 14: 177–182.
Mason GF, Petrakis IL, de Graaf RA, Gueorguieva E, Coric V, Epperson CN et al (2006). Cortical gamma-amino butyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry 59: 85–93.
McKenna M (2007). The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res 58: 3347–3358.
Mendelson JH, Woods BT, Chiu TM, Mello NK, Lukas SE, Teoh SK et al (1990). In vivo proton magnetic resonance spectroscopy of alcohol in human brain. Alcohol 7: 443–447.
Meyerhoff DJ, Durazzo TC (2008). Proton magnetic resonance spectroscopy in alcohol use disorders: a potential new endophenotype? Alcohol Clin Exp Res 32: 1146–1158.
Meyerhoff DJ, Rooney WD, Tokumitsu T, Weiner MW (1996). Evidence of multiple ethanol pools in the brain: an in vivo proton magnetization transfer study. Alcohol Clin Exp Res 20: 1283–1288.
Miller WR, Del Boca FC (1994). Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl 12: 112–118.
Miller WR, Tonigan JS, Longabaugh R (1995). The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Averse Consequence of Alcohol Abuse. Project MATCH Monograph Series: Rockville, MD.
Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C (2008). Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn Reson Med 60: 964–969.
Ongur D, Jensen J, Prescot A, Stork C, Lundy M, Cohen B et al (2008). Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry 64: 718–726.
Provencher SW (2001). Automatic quantitation of localized in vivo1H spectra with LCModel NMR in Biomedicine, Special Issue: NMR Spectroscopy Quantitation 14: 260–264.
Rossetti Z, Carboni S, Fadda F (1999). Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawl. Neuroscience 93: 1135–1140.
Rossetti ZL, Carboni S (1995). Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol 283: 177–183.
Rourke S, Loberg I (1996). Neurobehavioral correlates of alcoholism. In: Grant I, Adams K (eds). Neuropsychological Assessment of Neuropsychiatric Disorders. Oxford, New York.
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E et al (2005). Effects of ketamine in anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162: 394–396.
Schweinsburg BC, Taylor MJ, Alhassoon OM, Videen JS, Brown GG, Patterson TL et al (2001). Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin Exp Res 25: 924–934.
Szumlinski KK, Diab ME, Friedman R, Henze L, Lominac K, Bowers S (2007). Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology 190: 415–431.
Théberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J et al (2002). Glutamate and glutamine measured with 4.0 T Proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry 159: 1944–1946.
Tsai G, Coyle J (1998). The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med 49: 173–184.
Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L et al (2010). Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 67: 1069–1077.
US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration (2002). Results from the 2001 National Household Survey on Drug Abuse: Volume I. Summary of National Findings (Office of Applied Studies, NHSDA Series H-17 ed.) (BKD461, SMA 02-3758) US Government Printing Office: Washington, DC.
Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, Mayer AR (2011). A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. J Neurotrauma 28: 1–11.
Zhu W, Bie B, Pan ZZ (2007). Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci 27: 289–298.
Acknowledgements
This paper was supported by grants to Dr Thoma (PI: K23AA016544 & R21AA0173134) from the National Institute on Alcohol Abuse and Alcoholism and by funding from the Mind Research Network (DE-FG02-99ER62764).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Thoma, R., Mullins, P., Ruhl, D. et al. Perturbation of the Glutamate–Glutamine System in Alcohol Dependence and Remission. Neuropsychopharmacol 36, 1359–1365 (2011). https://doi.org/10.1038/npp.2011.20
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2011.20
Keywords
This article is cited by
-
Smoking, tobacco dependence, and neurometabolites in the dorsal anterior cingulate cortex
Molecular Psychiatry (2023)
-
J-difference GABA-edited MRS reveals altered cerebello-thalamo-cortical metabolism in patients with hepatic encephalopathy
Metabolic Brain Disease (2023)
-
Differential Effects of Chronic Ethanol Use on Mouse Neuronal and Astroglial Metabolic Activity
Neurochemical Research (2023)
-
Blood glutamine synthetase signaling in alcohol use disorder and racial disparity
Translational Psychiatry (2022)
-
Glutamine and GABA alterations in cingulate cortex may underlie alcohol drinking in a rat model of co-occurring alcohol use disorder and schizophrenia: an 1H-MRS study
Schizophrenia (2022)