Abstract
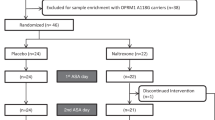
The wars in Iraq and Afghanistan are associated with high rates of post-traumatic stress disorder (PTSD) and comorbid alcohol use disorders. The pharmacotherapy of these comorbid conditions has received relatively little study. The current study compared the serotonin uptake inhibitor, paroxetine, to the norepinephrine uptake inhibitor, desipramine. It also evaluated the adjunctive efficacy of the Food and Drug Administration (FDA)-approved alcoholism pharmacotherapy, naltrexone, relative to placebo. Four groups of predominately male veterans (n=88) meeting current diagnostic criteria for both alcohol dependence (AD) and PTSD were randomly assigned under double-blind conditions to one of four groups: paroxetine+naltrexone; paroxetine+placebo; desipramine+naltrexone; desipramine+placebo. Main outcome measures included standardized scales that assessed symptoms of PTSD and alcohol consumption. Paroxetine did not show statistical superiority to desipramine for the treatment of PTSD symptoms. However, desipramine was superior to paroxetine with respect to study retention and alcohol use outcomes. Naltrexone reduced alcohol craving relative to placebo, but it conferred no advantage on drinking use outcomes. Although the serotonin uptake inhibitors are the only FDA-approved medications for the treatment of PTSD, the current study suggests that norepinephrine uptake inhibitors may present clinical advantages when treating male veterans with PTSD and AD. However, naltrexone did not show evidence of efficacy in this population. This study was registered with ClinicalTrials.gov, registration number NCT00338962 and URL: http://clinicaltrials.gov/ct2/show/NCT00338962?term=desipramine+AND+alcohol+dependence+AND+depression&recr=Closed&rank=1.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anton RF, Moak DH, Latham PK (1996). The obsessive compulsive drinking scale: a new method of assessing outcome in alcoholism treatment studies (published erratum appears in Arch Gen Psychiatry 1996 Jul;53(7):576). Arch Gen Psychiatry 53: 225–231.
Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM et al (2006). Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. [see comment]. JAMA 295: 2003–2017.
Berger W, Mendlowics MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR et al (2009). Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 33: 169–180.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G et al (1990). A clinician rating scale for assessing current and lifetime PTSD: the CAPS-1. Behav Ther 13: 187–188.
Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR et al (2000). Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA 283: 1837–1844.
Brady K, Sonne S, Roberts J (1995). Sertraline treatment of comorbid post traumatic stress disorder and alcohol dependence. J Clin Psychiatry 56: 502–505.
Brady KT, Sinha R (2005). Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. [see comment]. Am J Psychiatry 162: 1483–1493.
Brief DJ, Bollinger AR, Vielhauer MJ, Berger-Greenstein JA, Morgan EE, Brady SM et al (2004). Understanding the interface of HIV, trauma, post-traumatic stress disorder, and substance use and its implications for health outcomes. AIDS Care 16: S97–S120.
Brunner E, Domhof S, Langer F (2002). Nonparametric Analysis of Longitudinal Data in Factorial Experiments. John Wiley & Sons: New York, NY.
Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ (1998). Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 93: 713–727.
Charney DS, Heninger GR (1985). Noradrenergic function and the mechanism of action of antianxiety treatment. The effect of long-term imipramine treatment. Arch Gen Psychiatry 42: 473–481.
Charney DS, Heninger GR (1986). Alpha 2-adrenergic and opiate receptor blockade. Synergistic effects on anxiety in healthy subjects. Arch Gen Psychiatry 43: 1037–1041.
Davidson JR, Baldwin D, Stein DJ, Kuper E, Benattia I, Ahmed S et al (2006). Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Arch Gen Psychiatry 63: 1158–1165.
Erbes C, Westermeyer J, Engdahl B, Johnsen E (2007). Post-traumatic stress disorder and service utilization in a sample of service members from Iraq and Afghanistan. Mil Med 172: 359–363.
Fear NT, Jones M, Murphy D, Hull L, Sundin J, Iversen AC et al (2010). What are the consequences of deployment to Iraq and Afghanistan on the mental health of the UK armed forces? A cohort study. Lancet 375: 1783–1797.
First MB, Spitzer RL, Gibbon M, Williams JBW (1996). Structured Clinical Interview for DSM-IV Axis I Disorders (Patient Edition) (SCID-P). Biometric Research, New York State Psychiatric Institute, New York, NY.
Foa E, Keane TM, Friedman MJ (2000). The Effective Treatments for PTSD. The Guilford Press: New York.
Frank E, Thase ME (1999). Natural history and preventative treatment of recurrent mood disorders. Ann Rev Med 50: 453–468.
Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM (2007). Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry 68: 711–720.
Gueorguieva R, Krystal J (2004). Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 61: 310–317.
Hertzberg MA, Butterfield MI, Feldman ME, Beckham JC, Sutherland SM, Connor KM et al (1999). A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry 45: 1226–1229.
Hoge CW, Auchterlonie JL, Milliken CS (2006). Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA 295: 1023–1032.
Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL (2004). Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 351: 13–22.
Johnson SD (2008). Substance use, post-traumatic stress disorder and violence. Curr Opin Psychiatry 21: 242–246.
Keane TM, Gerardi RJ, Lyons JA, Wolfe J (1988). The interrelationship of substance abuse and posttraumatic stress disorder: epidemiological and clinical considerations. Recent Dev Alcohol 6: 27–48.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060.
Kosten TR, Krystal J (1988). Biological mechanisms in posttraumatic stress disorder: Relevance for substance abuse. Recent Dev Alcohol 6: 49–68.
Kranzler H, Burleson J, Brown J, Babor T (1996). Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res 20: 1534–1541.
Krystal J, Neumeister A (2009). Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resiliance. Brain Res 1293: 13–23.
Krystal JH, Cramer J, Krol W, Kirk G, Rosenheck R (2001). Naltrexone in the treatment of alcohol dependence. N Engl J Med 345: 1734–1739.
Levine J, Schooler NR (1986). Strategies for analyzing side effect data from SAFTEE: a workshop held fall 1985 in Rockville, Maryland. Psychopharmacol Bulletin 22: 343–356.
Marshall RD, Beebe KL, Oldham M, Zaninelli R (2001). Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 158: 1982–1988.
Mason BJ, Kocsis JH, Ritvo EC, Cutler RB (1996). A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression [see comments]. JAMA 275: 761–767.
Pae CU, Lim HK, Ajwani N, Lee C, Patkar AA (2007). Extended-release formulation of venlafaxine in the treatment of post-traumatic stress disorder. Expert Rev Neurother 7: 603–616.
Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Ralevski E et al (2006). Naltrexone and disulfiram in patients with alcohol dependence and post traumatic stress disorder. Biol Psychiatry 60: 777–783.
Pettinati HM (2001). The use of selective serotonin reuptake inhibitors in treating alcoholic subtypes. J Clin Psychiatry 62 Suppl: 26–31.
Pettinati HM (2004). Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry 56: 785–792.
Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D et al (2007). A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. [see comment]. Biol Psychiatry 61: 928–934.
Read JP, Brown PJ, Kahler CW (2004). Substance use and posttraumatic stress disorders: symptom interplay and effects on outcome. Addict Behav 29: 1665–1672.
Reist C, Kauffmann CD, Haier RJ, Sangdahl C, DeMet EM, Chicz-DeMet A et al (1989). A controlled trial of desipramine in 18 men with posttraumatic stress disorder. Am J Psychiatry 146: 513–516.
Richelson E (2003). Interactions of antidepressants with neurotransmitter transporters and receptors and their clinical relevance. J Clin Psychiatry 45: 1037–1041.
Riggs DS, Rukstalis M, Volpicelli JR, Kalmanson D, Foa EB (2003). Demographic and social adjustment characteristics of patients with comorbid posttraumatic stress disorder and alcohol dependence: potential pitfalls to PTSD treatment. Addict Behav 28: 1717–1730.
Rosen MI, Kosten TR, Kreek MJ (1999). The effects of naltrexone maintenance on the response to yohimbine in healthy volunteers. Biol Psychiatry 45: 1636–1645.
Rothbaum BO, Killeen TK, Davidson JR, Brady KT, Connor KM, Heekin MH (2008). Placebo-controlled trial of risperidone augmentation for selective serotonin reuptake inhibitor-resistant posttraumatic stress disorder. J Clin Psychiatry 69: 520–525.
Saladin ME, Brady KT, Dansky BS, Kilpatrick DG (1995). Understanding comorbidity between PTSD and substance use disorders: two preliminary investigations. Addict Behav 20: 643–655.
Seal KH, Bertenthal D, Miner CR, Sen S, Marmar C (2007). Bringing the war back home: mental health disorders among 103,788 US veterans returning from Iraq and Afghanistan seen at Department of Veterans Affairs facilities. Arch Intern Med 167: 476–482.
Skinner HA, Horn JL (1984). Alcohol Dependence Scale (ADS): User's Guide. Addiction Research Foundation: Toronto.
Smajkic A, Weine S, Djuric-Bijedic Z, Boskailo E, Lewis J, Pavkovic I (2001). Sertraline, paroxetine, and venlafaxine in refugee posttraumatic sress disorder with depression symptoms. J Trauma Stress 14: 445–452.
Sobell LC, Sobell MB (1992). Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. Measuring Alcohol Consumption: Psychosoical and biological methods. Litten, RZ and Allen, J. Humana Press: Totowa, NJ. pp 41–72.
Spivak B, Strous RD, Shaked G, Shabash E, Kotler M, Weizman A (2006). Reboxetine vs fluvoxamine in the treatment of motor vehicle accident-related posttraumatic stress disorder: a double-blind, fixed-dosage, controlled trial. J Clin Psychopharmacol 26: 152–156.
Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD (2001). Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry 62: 860–868.
Van der Kolk B (2004). Psychobiology of posttraumatic stress disorder. Textbook of Biological Psychiatry. Panksepp, J. Wiley-Liss: New York. pp 319–344.
Acknowledgements
This study was conducted with the invaluable help of the VA VISN I MIRECC Study Group: Department of Psychiatry, Bedford VAMC: Marylee Losardo, MSPA, Barbara E Rofman, RN, MS; Department of Psychology, Bedford VAMC: Charles E Drebing, PhD; Department of Psychiatry, VA CT Healthcare, West Haven Campus: Kathryn Keegan, RN, Diana Limoncelli, BA, Colette McHugh-Strong, JD, Alison Oville, BA, Christine Sicignano, BA, J Serrita Jane, PhD, Erin O’Brien, PsyD. Support was provided by VISN I Mental Illness Research Education and Clinical Center (MIRECC; PI, Rounsaville), the VA Alcohol Center (PI, Krystal), and Clinical Neuroscience Division of the VA National Center for PTSD (PI, Krystal).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Ismene L Petrakis, Dr Elizabeth Ralevski, Dr Nitigna Desai, Dr Louis Trevisan, and Dr Ralitza Gueorguieva declare no conflict of interest. Dr John H Krystal (during the period 2008–2011) has served as a scientific consultant to the following companies (The Individual Consultant Agreements listed below are less than $10 000 per year): Aisling Capital, LLC AstraZeneca Pharmaceuticals, Biocortech, Brintnall & Nicolini, Easton Associates, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Merz Pharmaceuticals, MK Medical Communications, F Hoffmann-La Roche, SK Holdings, Sunovion Pharmaceuticals, Takeda Industries, Teva Pharmaceutical Industries. He is on the Scientific Advisory Board for the following companies: Abbott Laboratories, Bristol-Myers Squibb, Eisai, Eli Lilly, Forest Laboratories, Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, Pfizer Pharmaceuticals, Shire Pharmaceuticals. He holds less than $150 in exercisable warrant options with Tetragenex Pharmaceuticals. He is on the Board of Directors: Coalition for Translational Research in Alcohol and Substance Use Disorders. He is President Elect: American College of Neuropsychopharmacology. He is the principal investigator of a multicenter study in which Janssen Research Foundation has provided drug and some support to the Department of Veterans Affairs. He is on the Editorial Board, Editor of Biological Psychiatry (Income Greater than $10 000). He has Patents and Inventions: 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent number: 5 447 948, 5 September 1995; I am a co-inventor with Dr Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1). Intranasal Administration of Ketamine to Treat Depression (pending).
Rights and permissions
About this article
Cite this article
Petrakis, I., Ralevski, E., Desai, N. et al. Noradrenergic vs Serotonergic Antidepressant with or without Naltrexone for Veterans with PTSD and Comorbid Alcohol Dependence. Neuropsychopharmacol 37, 996–1004 (2012). https://doi.org/10.1038/npp.2011.283
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2011.283
Keywords
This article is cited by
-
Selective serotonin reuptake inhibitors in the treatment of depression, anxiety, and post-traumatic stress disorder in substance use disorders: a Bayesian meta-analysis
European Journal of Clinical Pharmacology (2022)
-
Dealing With Complexity and Comorbidity: Comorbid PTSD and Substance Use Disorders
Current Treatment Options in Psychiatry (2019)
-
Augmenting Treatment for Posttraumatic Stress Disorder and Co-Occurring Conditions with Oxytocin
Current Treatment Options in Psychiatry (2019)
-
Old Friends, immunoregulation, and stress resilience
Pflügers Archiv - European Journal of Physiology (2019)
-
Therapeutic challenges for concurrent ethanol and nicotine consumption: naltrexone and varenicline fail to alter simultaneous ethanol and nicotine intake by female alcohol-preferring (P) rats
Psychopharmacology (2019)