Abstract
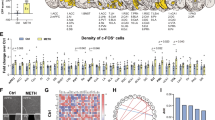
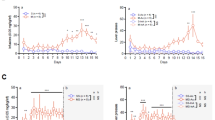
Methamphetamine affects the hippocampus, a brain region crucial for learning and memory, as well as relapse to drug seeking. Rats self-administered methamphetamine for 1 h twice weekly (intermittent-short-I-ShA), 1 h daily (limited-short-ShA), or 6 h daily (extended-long-LgA) for 22 sessions. After 22 sessions, rats from each access group were withdrawn from self-administration and underwent spatial memory (Y-maze) and working memory (T-maze) tests followed by extinction and reinstatement to methamphetamine seeking or received one intraperitoneal injection of 5-bromo-2′-deoxyuridine (BrdU) to label progenitors in the hippocampal subgranular zone (SGZ) during the synthesis phase. Two-hour-old and 28-day-old surviving BrdU-immunoreactive cells were quantified. I-ShA rats performed better on the Y-maze and had a greater number of 2-h-old SGZ BrdU cells than nondrug controls. LgA rats, but not ShA rats, performed worse on the Y- and T-maze and had a fewer number of 2-h-old SGZ BrdU cells than nondrug and I-ShA rats, suggesting that new hippocampal progenitors, decreased by methamphetamine, were correlated with impairment in the acquisition of new spatial cues. Analyses of addiction-related behaviors after withdrawal and extinction training revealed methamphetamine-primed reinstatement of methamphetamine-seeking behavior in all three groups (I-ShA, ShA, and LgA), and this effect was enhanced in LgA rats compared with I-ShA and ShA rats. Protracted withdrawal from self-administration enhanced the survival of SGZ BrdU cells, and methamphetamine seeking during protracted withdrawal enhanced Fos expression in the dentate gyrus and medial prefrontal cortex in LgA rats to a greater extent than in ShA and I-ShA rats. These results indicate that changes in the levels of the proliferation and survival of hippocampal neural progenitors and neuronal activation of hippocampal granule cells predict the effects of methamphetamine self-administration (limited vs extended access) on cognitive performance and relapse to drug seeking and may contribute to the impairments that perpetuate the addiction cycle.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Altman J, Das GD (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335.
Black AH, Nadel L, O’Keefe J (1977). Hippocampal function in avoidance learning and punishment. Psychol Bull 84: 1107–1129.
Brown EE, Robertson GS, Fibiger HC (1992). Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci 12: 4112–4121.
Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003). Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467: 1–10.
Burgess N, Maguire EA, O’Keefe J (2002). The human hippocampus and spatial and episodic memory. Neuron 35: 625–641.
Cameron HA, McKay RD (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435: 406–417.
Canales JJ (2007). Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosci 257: 261–270.
Canales JJ (2010). Comparative neuroscience of stimulant-induced memory dysfunction: role for neurogenesis in the adult hippocampus. Behav Pharmacol 21: 379–393.
Ciccocioppo R, Sanna PP, Weiss F (2001). Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA 98: 1976–1981.
Comer SD, Collins ED, Fischman MW (1997). Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol 8: 677–690.
Conrad CD, Galea LA, Kuroda Y, McEwen BS (1996). Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 110: 1321–1334.
Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP (1995). The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 120: 392–399.
Cryan JF, Hoyer D, Markou A (2003). Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry 54: 49–58.
Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA (2003). Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol 460: 563–572.
Eichenbaum H, Otto T, Cohen NJ (1992). The hippocampus—what does it do? Behav Neural Biol 57: 2–36.
Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ (2000). Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA 97: 7579–7584.
Feltenstein MW, See RE (2008). The neurocircuitry of addiction: an overview. Br J Pharmacol 154: 261–274.
Garcia-Fuster MJ, Flagel SB, Mahmood ST, Mayo LM, Thompson RC, Watson SJ et al (2011). Decreased proliferation of adult hippocampal stem cells during cocaine withdrawal: possible role of the cell fate regulator FADD. Neuropsychopharmacology 36: 2303–2317.
George O, Mandyam CD, Wee S, Koob GF (2008). Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33: 2474–2482.
Gerlai R (1998). A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav Brain Res 95: 91–101.
Gerlai R (2001). Behavioral tests of hippocampal function: simple paradigms complex problems. Behav Brain Res 125: 269–277.
Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999). Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260–265.
Graybiel AM, Moratalla R, Robertson HA (1990). Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci USA 87: 6912–6916.
Gross NB, Marshall JF (2009). Striatal dopamine and glutamate receptors modulate methamphetamine-induced cortical Fos expression. Neuroscience 161: 1114–1125.
Hart CL, Ward AS, Haney M, Nasser J, Foltin RW (2003). Methamphetamine attenuates disruptions in performance and mood during simulated night-shift work. Psychopharmacology (Berl) 169: 42–51.
Hildebrandt K, Teuchert-Noodt G, Dawirs RR (1999). A single neonatal dose of methamphetamine suppresses dentate granule cell proliferation in adult gerbils which is restored to control values by acute doses of haloperidol. J Neural Transm 106: 549–558.
Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T (2006). Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci USA 103: 8523–8527.
Hiranita T, Nawata Y, Sakimura K, Yamamoto T (2008). Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology 55: 1300–1306.
Holscher C, Schmidt WJ (1994). Quinolinic acid lesion of the rat entorhinal cortex pars medialis produces selective amnesia in allocentric working memory (WM), but not in egocentric WM. Behav Brain Res 63: 187–194.
Hope B, Kosofsky B, Hyman SE, Nestler EJ (1992). Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci USA 89: 5764–5768.
Jessberger S, Clark RE, Broadbent NJ, Clemenson Jr GD, Consiglio A, Lie DC et al (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem 16: 147–154.
Johnson BA, Ait-Daoud N, Wells LT (2000). Effects of isradipine, a dihydropyridine-class calcium channel antagonist, on D-methamphetamine-induced cognitive and physiological changes in humans. Neuropsychopharmacology 22: 504–512.
Johnson BA, Roache JD, Ait-Daoud N, Wallace C, Wells LT, Wang Y (2005). Effects of isradipine on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition. Psychopharmacology (Berl) 178: 296–302.
Jones HE, Garrett BE, Griffiths RR (2001). Reinforcing effects of oral cocaine: contextual determinants. Psychopharmacology (Berl) 154: 143–152.
Kalivas PW, McFarland K (2003). Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168: 44–56.
Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002). The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115: 97–105.
Kim WR, Christian K, Ming GL, Song H (2011). Time-dependent involvement of adult-born dentate granule cells in behavior. Behav Brain Res; e-pub ahead of print.
Kim YT, Lee JJ, Song HJ, Kim JH, Kwon DH, Kim MN et al (2010). Alterations in cortical activity of male methamphetamine abusers performing an empathy task: fMRI study. Hum Psychopharmacol 25: 63–70.
King VR, Corwin JV (1992). Spatial deficits and hemispheric asymmetries in the rat following unilateral and bilateral lesions of posterior parietal or medial agranular cortex. Behav Brain Res 50: 53–68.
Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A et al (2004). Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27: 739–749.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Lalonde R (2002). The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26: 91–104.
Lasseter HC, Xie X, Ramirez DR, Fuchs RA (2010). Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience 171: 830–839.
Leuner B, Gould E, Shors TJ (2006). Is there a link between adult neurogenesis and learning? Hippocampus 16: 216–224.
Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G (2011). Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333: 353–357.
Mandyam CD, Harburg GC, Eisch AJ (2007a). Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 146: 108–122.
Mandyam CD, Norris RD, Eisch AJ (2004). Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res 76: 783–794.
Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF (2008). Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry 64: 958–965.
Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF (2007b). Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci 27: 11442–11450.
Moratalla R, Elibol B, Vallejo M, Graybiel AM (1996). Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron 17: 147–156.
Morgan D, Roberts DC (2004). Sensitization to the reinforcing effects of cocaine following binge-abstinent self-administration. Neurosci Biobehav Rev 27: 803–812.
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683.
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF (2000). Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20: 798–805.
Nestler EJ (2001). Molecular neurobiology of addiction. Am J Addict 10: 201–217.
Nieto-Escamez FA, Sanchez-Santed F, de Bruin JP (2002). Cholinergic receptor blockade in prefrontal cortex and lesions of the nucleus basalis: implications for allocentric and egocentric spatial memory in rats. Behav Brain Res 134: 93–112.
Noonan MA, Bulin SE, Fuller DC, Eisch AJ (2010). Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci 30: 304–315.
Noonan MA, Choi KH, Self DW, Eisch AJ (2008). Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci 28: 2516–2526.
Parrott AC, Gibbs A, Scholey AB, King R, Owens K, Swann P et al (2011). MDMA and methamphetamine: some paradoxical negative and positive mood changes in an acute dose laboratory study. Psychopharmacology (Berl) 215: 527–536.
Paxinos G, Watson C (1997). The Rat Brain in Stereotaxic Coordinates, 3rd edn Academic Press: San Diego.
Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE (2006). The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci 24: 2089–2097.
Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011). Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36: 782–792.
Ricoy UM, Martinez Jr JL (2009). Local hippocampal methamphetamine-induced reinforcement. Front Behav Neurosci 3: 47.
Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H et al (1999). Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci 2: 898–905.
Rogers JL, De Santis S, See RE (2008). Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 199: 615–624.
Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K et al (1999). Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 20: 322–339.
Ruskin DN, Marshall JF (1994). Amphetamine- and cocaine-induced fos in the rat striatum depends on D2 dopamine receptor activation. Synapse 18: 233–240.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809.
Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH et al (2010). Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage 50: 1392–1401.
Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH et al (2007). Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17: 275–297.
Scoville WB, Milner B (2000). Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci 12: 103–113.
Shen F, Meredith GE, Napier TC (2006). Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci 26: 11041–11051.
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410: 372–376.
Silber BY, Croft RJ, Papafotiou K, Stough C (2006). The acute effects of d-amphetamine and methamphetamine on attention and psychomotor performance. Psychopharmacology (Berl) 187: 154–169.
Silverman K, Kirby KC, Griffiths RR (1994). Modulation of drug reinforcement by behavioral requirements following drug ingestion. Psychopharmacology (Berl) 114: 243–247.
Simon SL, Dean AC, Cordova X, Monterosso JR, London ED (2010). Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs 71: 335–344.
Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR (2005). Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 182: 186–193.
Sudai E, Croitoru O, Shaldubina A, Abraham L, Gispan I, Flaumenhaft Y et al (2011). High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict Biol 16: 251–260.
Tani K, Iyo M, Matsumoto H, Kawai M, Suzuki K, Iwata Y et al (2001). The effects of dentate granule cell destruction on behavioural activity and Fos protein expression induced by systemic methamphetamine in rats. Br J Pharmacol 134: 1411–1418.
Teuchert-Noodt G, Dawirs RR, Hildebrandt K (2000). Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm 107: 133–143.
Ursin R, Ursin H, Olds J (1966). Self-stimulation of hippocampus in rats. J Comp Physiol Psychol 61: 353–359.
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96: 13427–13431.
Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL (2001). Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292: 1175–1178.
Wall PM, Messier C (2001). The hippocampal formation—orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res 127: 99–117.
Yan Y, Yamada K, Nitta A, Nabeshima T (2007). Transient drug-primed but persistent cue-induced reinstatement of extinguished methamphetamine-seeking behavior in mice. Behav Brain Res 177: 261–268.
Yuan CJ, Quiocho JM, Kim A, Wee S, Mandyam CD (2011). Extended access methamphetamine decreases immature neurons in the hippocampus which results from loss and altered development of neural progenitors without altered dynamics of the S-phase of the cell cycle. Pharmacol Biochem Behav 100: 98–108.
Zhou LF, Zhu YP (2006). Changes of CREB in rat hippocampus, prefrontal cortex and nucleus accumbens during three phases of morphine induced conditioned place preference in rats. J Zhejiang Univ Sci B 7: 107–113.
Acknowledgements
The study was supported by funds from the National Institute on Drug Abuse (DA022473 to CDM, DA010072 to GFK, and summer research fellowship to AHS), Pearson Center for Alcoholism and Addiction Research to GFK, NIH/Eunice Kennedy Shriver National Institute of Child Health & Human Development (T35HD064385 to PR), and Skaggs School of Pharmacy and Pharmaceutical Sciences (Skaggs Scholarship to CJY). We acknowledge the excellent technical assistance of Roxanne Kotzebue and Cameron Muhlfeld from the independent study program at the University of California, San Diego, and the San Diego State University, respectively, and Siddharth Iyengar and Wednesday Bushong from the Life Sciences Summer Internship Program at The Scripps Research Institute for assistance with immunohistochemistry and the cognitive behavior study. We appreciate the technical support of Elena Crawford and Robert Lintz and the editorial assistance of Michael Arends. The authors report no biomedical financial interests or potential conflict of interest. The publication number 21357 is from The Scripps Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that, except for the income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Recinto, P., Samant, A., Chavez, G. et al. Levels of Neural Progenitors in the Hippocampus Predict Memory Impairment and Relapse to Drug Seeking as a Function of Excessive Methamphetamine Self-Administration. Neuropsychopharmacol 37, 1275–1287 (2012). https://doi.org/10.1038/npp.2011.315
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2011.315
Keywords
This article is cited by
-
The Role of Non-coding RNAs in Methamphetamine-Induced Neurotoxicity
Cellular and Molecular Neurobiology (2023)
-
Effects of Moderate-Intensity Interval Training on Gene Expression and Antioxidant Status in the Hippocampus of Methamphetamine-Dependent Rats
Neurotoxicity Research (2022)
-
Footshock-Induced Abstinence from Compulsive Methamphetamine Self-administration in Rat Model Is Accompanied by Increased Hippocampal Expression of Cannabinoid Receptors (CB1 and CB2)
Molecular Neurobiology (2022)
-
Prevention of relapse to methamphetamine self-administration by environmental enrichment: involvement of glucocorticoid receptors
Psychopharmacology (2022)
-
Cannabidiol promotes neurogenesis in the dentate gyrus during an abstinence period in rats following chronic exposure to methamphetamine
Metabolic Brain Disease (2021)