Abstract
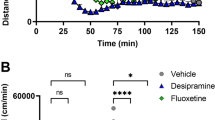
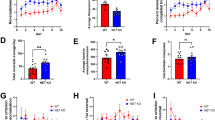
Prepulse inhibition (PPI) deficits are among the most reproducible phenotypic markers found in schizophrenic patients. We recently reported that nisoxetine, a selective norepinephrine transporter (NET) inhibitor, reversed the PPI deficits that have been identified in dopamine transporter (DAT) knockout (KO) mice. However, the mechanisms underlying nisoxetine-induced PPI recovery in DAT KO mice were unclear in previous experiments. To clarify these mechanisms, PPI was tested after microinjections of nisoxetine into the medial prefrontal cortex (mPFc) or nucleus accumbens (NAc) in wildtype (WT) and DAT KO mice. c-Fos immunohistochemistry provided an indicator of neural activation. Multiple-fluorescent-labeling procedures and the retrograde tracer fluorogold were employed to identify nisoxetine-activated neurons and circuits. Systemic nisoxetine activated the mPFc, the NAc shell, the basolateral amygdala, and the subiculum. Infusions of nisoxetine into the mPFc reversed PPI deficits in DAT KO mice, but produced no changes in WT mice, while infusion of nisoxetine into the NAc had no effect on PPI in both WT and DAT KO mice. Experiments using multiple-fluorescent labeling/fluorogold revealed that nisoxetine activates presumed glutamatergic pyramidal cells that project from the mPFc to the NAc. Activated glutamatergic projections from the mPFc to the NAc appear to have substantial roles in the ability of a NET inhibitor to normalize PPI deficits in DAT KO. Thus, this data suggest that selective NET inhibitors such as nisoxetine might improve information processing deficits in schizophrenia via regulation of cortico-subcortical neuromodulation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Arime Y, Yamashita M, Fukushima S, Shen H-W, Hagino Y, Hall FS et al (2006). Norepinephrine transporter blockade reversed the prepulse inhibition deficits in dopamine transporter knockout mice. The Society for Neuroscience 36th Annual Meeting, Atlanta, USA.
Bisler S, Schleicher A, Gass P, Stehle JH, Zilles K, Staiger JF (2002). Expression of c-Fos, ICER, Krox-24 and JunB in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. J Chem Neuroanat 23: 187–198.
Braff DL, Geyer MA, Swerdlow NR (2001). Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 156: 234–258.
Brog JS, Salyapongse A, Deutch AY, Zahm DS (1993). The patterns of afferent innervation of the core and shell in the ″accumbens″ part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338: 255–278.
Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH et al (2002). Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27: 699–711.
Carboni E, Tanda GL, Frau R, Di Chiara G (1990). Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem 55: 1067–1070.
Carr DB, O′Donnell P, Card JP, Sesack SR (1999). Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci 19: 11049–11060.
Deutch AY, Duman RS (1996). The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: cellular localization and pharmacological characterization. Neuroscience 70: 377–389.
Ellenbroek BA, Budde S, Cools AR (1996). Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience 75: 535–542.
Fendt M, Schwienbacher I, Koch M (2000). Amygdaloid N-methyl-D-aspartate and gamma-aminobutyric acid(A) receptors regulate sensorimotor gating in a dopamine-dependent way in rats. Neuroscience 98: 55–60.
Gehlert DR, Thompson LK, Hemrick-Luecke SK, Shaw J (2008). Monoaminergic compensation in the neuropeptide Y deficient mouse brain. Neuropeptides 42: 367–375.
Geyer MA (2006). Are cross-species measures of sensorimotor gating useful for the discovery of procognitive cotreatments for schizophrenia? Dialogues Clin Neurosci 8: 9–16.
Geyer MA, Dulawa SC (2003). Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci Chapter 8, Unit 8 17.
Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC et al (2007). Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry 64: 1115–1122.
Grace AA, Floresco SB, Goto Y, Lodge DJ (2007). Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30: 220–227.
Hasue RH, Shammah-Lagnado SJ (2002). Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol 454: 15–33.
Ishii D, Matsuzawa D, Kanahara N, Matsuda S, Sutoh C, Ohtsuka H et al (2010). Effects of aripiprazole on MK-801-induced prepulse inhibition deficits and mitogen-activated protein kinase signal transduction pathway. Neurosci Lett 471: 53–57.
Iversen SD, Iversen LL (2007). Dopamine: 50 years in perspective. Trends Neurosci 30: 188–193.
Jentsch JD, Roth RH (1999). The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20: 201–225.
Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM et al (2007). Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry 64: 633–647.
Lewis DA, Lieberman JA (2000). Catching up on schizophrenia: natural history and neurobiology. Neuron 28: 325–334.
Marek GJ, Behl B, Bespalov AY, Gross G, Lee Y, Schoemaker H (2010). Glutamatergic (N-methyl-D-aspartate receptor) hypofrontality in schizophrenia: too little juice or a miswired brain? Mol Pharmacol 77: 317–326.
Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL (2006). Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA 103: 2938–2942.
Meredith GE (1999). The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci 877: 140–156.
Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002). Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22: 389–395.
Mueser KT, McGurk SR (2004). Schizophrenia. Lancet 363: 2063–2072.
Ohashi K, Hamamura T, Lee Y, Fujiwara Y, Suzuki H, Kuroda S (2000). Clozapine- and olanzapine-induced Fos expression in the rat medial prefrontal cortex is mediated by beta-adrenoceptors. Neuropsychopharmacology 23: 162–169.
Paxinos GFK (2001). The mouse brain in stereotaxic coordinates 2nd edn. Academic Press: San Diego.
Ralph RJ, Caine SB (2005). Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther 312: 733–741.
Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA (2001). Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci 21: 305–313.
Schmajuk NA, Larrauri JA (2005). Neural network model of prepulse inhibition. Behav Neurosci 119: 1546–1562.
Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD (2000). Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol 420: 211–232.
Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H et al (2004). Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology 29: 1790–1799.
Shoemaker JM, Saint Marie RL, Bongiovanni MJ, Neary AC, Tochen LS, Swerdlow NR (2005). Prefrontal D1 and ventral hippocampal N-methyl-D-aspartate regulation of startle gating in rats. Neuroscience 135: 385–394.
Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R et al (1998). Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA 95: 7699–7704.
Stone EA, Zhang Y, John SM, Bing G (1991). c-Fos response to administration of catecholamines into brain by microdialysis. Neurosci Lett 133: 33–35.
Sumner BE, Cruise LA, Slattery DA, Hill DR, Shahid M, Henry B (2004). Testing the validity of c-fos expression profiling to aid the therapeutic classification of psychoactive drugs. Psychopharmacology (Berl) 171: 306–321.
Swerdlow NR, Geyer MA, Braff DL (2001). Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 156: 194–215.
Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL (2006). Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry 63: 1325–1335.
Tamminga CA (2006). The neurobiology of cognition in schizophrenia. J Clin Psychiatry 67 (Suppl 9): 9–13 discussion 36–42.
Wan FJ, Caine SB, Swerdlow NR (1996a). The ventral subiculum modulation of prepulse inhibition is not mediated via dopamine D2 or nucleus accumbens non-NMDA glutamate receptor activity. Eur J Pharmacol 314: 9–18.
Wan FJ, Swerdlow NR (1996b). Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res 722: 168–176.
Wright CI, Groenewegen HJ (1995). Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol 361: 383–403.
Yamamoto BK, Novotney S (1998). Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 71: 274–280.
Yamashita M, Fukushima S, Shen HW, Hall FS, Uhl GR, Numachi Y et al (2006). Norepinephrine transporter blockade can normalize the prepulse inhibition deficits found in dopamine transporter knockout mice. Neuropsychopharmacology 31: 2132–2139.
Zhang X, Bearer EL, Boulat B, Hall FS, Uhl GR, Jacobs RE (2010). Altered neurocircuitry in the dopamine transporter knockout mouse brain. PLoS One 5: e11506.
Acknowledgements
We thank Dr Takeshi Kaneko for the gift of PAG monoclonal antibody, Nozomi Okayasu for technical assistance, and Dr Taku Sato and Dr Shiho Miyazawa for assistance with statistical analysis. This work was supported in part by Scientific Research on Priority Areas-System study on higher-order brain functions and Research on Pathomechanisms of Brain Disorders, Core Research for Evolutional Science and Technology (CREST) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Global COE Program (Basic and Translational Research Center for Global Brain Science), MEXT, Japan, and through funding from the Intramural Research Program of the National Institute on Drug Abuse, NIH/DHHS, USA (GRU and FSH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Arime, Y., Kasahara, Y., Hall, F. et al. Cortico-Subcortical Neuromodulation Involved in the Amelioration of Prepulse Inhibition Deficits in Dopamine Transporter Knockout Mice. Neuropsychopharmacol 37, 2522–2530 (2012). https://doi.org/10.1038/npp.2012.114
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2012.114
Keywords
This article is cited by
-
AgRP neurons control structure and function of the medial prefrontal cortex
Molecular Psychiatry (2022)
-
Reduced Dopamine Transporter Functioning Induces High-Reward Risk-Preference Consistent with Bipolar Disorder
Neuropsychopharmacology (2014)
-
Impaired cliff avoidance reaction in dopamine transporter knockout mice
Psychopharmacology (2013)
-
Transgenic mouse models for ADHD
Cell and Tissue Research (2013)