Abstract
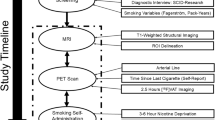
The primary objective of this project was to determine the α4β2* nicotinic acetylcholine receptor (nAChR) occupancy in human brain of a single low dose of varenicline (0.5 mg), and to explore the relationship between receptor occupancy by varenicline and tobacco withdrawal symptoms (*denoting other putative nAChR subunits). Otherwise healthy smokers (n=9) underwent two positron emission tomography (PET) sessions with the selective α4β2* radioligand 2-FA. For the PET sessions, participants received either a low dose of varenicline (0.5 mg) or matching placebo pill (double-blind, random order) before imaging. For both sessions, participants received bolus plus continuous infusions of 2-FA, were scanned for 1 h after allowing the radiotracer to reach a steady state, smoked to satiety, and were scanned for 2 more hours. We estimated the fractional receptor occupancy by a single dose of varenicline (0.5 mg) and the corresponding varenicline dissociation constant (KV), along with the effect of low-dose varenicline, pill placebo, and smoking-to-satiety on withdrawal rating scales. The data are compatible with 100% occupancy of α4β2* nAChRs by a single dose of varenicline, with a 90% lower confidence limit of 89% occupancy for the thalamus and brainstem. The corresponding 90% upper limit on effective KV with respect to plasma varenicline was 0.49 nM. Smoking to satiety, but not low-dose varenicline, significantly reduced withdrawal symptoms. Our findings demonstrate that low-dose varenicline results in saturation of α4β2* nAChRs in the thalamus and brainstem without reducing withdrawal symptoms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anderson DJ, Malysz J, Gronlien JH, El Kouhen R, Hakerud M, Wetterstrand C et al (2009). Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity alpha4beta2 subunit combination. Biochem Pharmacol 78: 844–851.
Benowitz NL, Jacob III P (1993). Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther 53: 316–323.
Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J et al (2009). Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol 12: 305–316.
Brody AL, Mandelkern MA, London ED, Khan A, Kozman D, Costello MR et al (2011). Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Arch Gen Psychiatry 68: 953–960.
Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D et al (2006). Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63: 907–915.
Buisson B, Bertrand D (2001). Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J Neurosci 21: 1819–1829.
Cahill K, Stead LF, Lancaster T (2011). Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev(2): CD006103.
Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG (2005). Revealing the multidimensional framework of the Minnesota nicotine withdrawal scale. Curr Med Res Opin 21: 749–760.
Changeux JP (2010). Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 11: 389–401.
Cox LS, Tiffany ST, Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3: 7–16.
Doll F, Dolci L, Valette H, Hinnen F, Vaufrey F, Guenther I et al (1999). Synthesis and nicotinic acetylcholine receptor in vivo binding properties of 2-fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine: a new positron emission tomography ligand for nicotinic receptors. J Med Chem 42: 2251–2259.
Fagerstrom KO (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3: 235–241.
Fowler CD, Arends MA, Kenny PJ (2008). Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol 19: 461–484.
Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y et al (2011). Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry 68: 516–526.
Harpsoe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T (2011). Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J Neurosci 31: 10759–10766.
Hatsukami D, McBride C, Pirie P, Hellerstedt W, Lando H (1991). Effects of nicotine gum on prevalence and severity of withdrawal in female cigarette smokers. J Subst Abuse 3: 427–440.
Hughes JR (2007a). Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 9: 315–327.
Hughes JR (2007b). Measurement of the effects of abstinence from tobacco: a qualitative review. Psychol Addict Behav 21: 127–137.
Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW (1991). Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry 48: 52–59.
Hughes JR, Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43: 289–294.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539.
Jackson KJ, Martin BR, Changeux JP, Damaj MI (2008). Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325: 302–312.
Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI (2009). The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther 331: 547–554.
Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL (2000). Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav 66: 553–558.
Kenny PJ, Markou A (2001). Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70: 531–549.
Lotfipour S, Arnold MM, Hogenkamp DJ, Gee KW, Belluzzi JD, Leslie FM (2011a). The monoamine oxidase (MAO) inhibitor tranylcypromine enhances nicotine self-administration in rats through a mechanism independent of MAO inhibition. Neuropharmacology 61: 95–104.
Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L et al (2009). Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry 66: 1244–1252.
Lotfipour S, Leonard G, Perron M, Pike B, Richer L, Seguin JR et al (2010). Prenatal exposure to maternal cigarette smoking interacts with a polymorphism in the alpha6 nicotinic acetylcholine receptor gene to influence drug use and striatum volume in adolescence. Mol Psychiatry 15: 6–8.
Lotfipour S, Mandelkern MA, Brody AL (2011b). Quantitative Molecular Imaging of Neuronal Nicotinic Acetylcholine Receptors in the Human Brain with A-85380 Radiotracers. Curr Med Imaging Rev 7: 107–112.
Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S et al (2010). Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry 67: 715–721.
Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I (2006). alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 70: 755–768.
Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS et al (2006). Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 34: 121–130.
Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC et al (2009). Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry 65: 144–149.
Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008). It is not ‘either/or’: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84: 329–342.
Quick MW, Lester RA (2002). Desensitization of neuronal nicotinic receptors. J Neurobiol 53: 457–478.
Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L (1996). Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature 380: 347–351.
Raybuck JD, Portugal GS, Lerman C, Gould TJ (2008). Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav Neurosci 122: 1166–1171.
Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA et al (2007). Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52: 985–994.
Rollema H, Shrikhande A, Ward KM, Tingley III FD, Coe JW, O’Neill BT et al (2010). Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol 160: 334–345.
Russell MA, Peto J, Patel UA (1974). The classification of smoking by factorial structure of motives. J R Stat Soc Ser A 137: 313–346.
Shiffman SM, Jarvik ME (1976). Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology 50: 35–39.
Shumway DA, Pavlova OA, Mukhin AG (2007). A simplified method for the measurement of nonmetabolized 2-[18F]F-A-85380 in blood plasma using solid-phase extraction. Nucl Med Biol 34: 221–228.
Sokolova E, Matteoni C, Nistri A (2005). Desensitization of neuronal nicotinic receptors of human neuroblastoma SH-SY5Y cells during short or long exposure to nicotine. Br J Pharmacol 146: 1087–1095.
Sorger D, Becker GA, Hauber K, Schildan A, Patt M, Birkenmeier G et al (2006). Binding properties of the cerebral alpha4beta2 nicotinic acetylcholine receptor ligand 2-[18F]fluoro-A-85380 to plasma proteins. Nucl Med Biol 33: 899–906.
Sorger D, Becker GA, Patt M, Schildan A, Grossmann U, Schliebs R et al (2007). Measurement of the alpha4beta2* nicotinic acetylcholine receptor ligand 2-[(18)F]Fluoro-A-85380 and its metabolites in human blood during PET investigation: a methodological study. Nucl Med Biol 34: 331–342.
Spielberger C (1983). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA.
Steer RA, Cavalieri TA, Leonard DM, Beck AT (1999). Use of the Beck Depression Inventory for Primary Care to screen for major depression disorders. Gen Hosp Psychiatry 21: 106–111.
Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S et al (2010). Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet 153B: 1350–1354.
Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing Jr CB et al (2007). A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther 29: 1027–1039.
Turner JR, Castellano LM, Blendy JA (2011). Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 13: 41–46.
Ueta I, Saito Y, Teraoka K, Miura T, Jinno K (2010). Determination of volatile organic compounds for a systematic evaluation of third-hand smoking. Anal Sci 26: 569–574.
West R, Baker CL, Cappelleri JC, Bushmakin AG (2008). Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 197: 371–377.
Acknowledgements
We thank Karen Ta, Jaime La Charite, Betram Nweke, Stuart Mirell, Van Cayetano, Chien Nguyen, Judah Farahi, Andrew Leuchter, Hans Rollema, Maziar Maghsoodnia, Alison Curtin, Ron Waldorf, Diep Seversen, and Josephine Ribe for their assistance on the study. This work was funded by Pfizer Pharmaceutical Corporation (Grant number: WS653344) (SL) and, in part, by T-32 NIMH post-doctoral fellowship (MH17140) (SL), NIDA (R01 DA20872) (ALB), and the Richard Metzner Chair in Clinical Neuropharmacology (ALB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Lotfipour, S., Mandelkern, M., Alvarez-Estrada, M. et al. A Single Administration of Low-Dose Varenicline Saturates α4β2* Nicotinic Acetylcholine Receptors in the Human Brain. Neuropsychopharmacol 37, 1738–1748 (2012). https://doi.org/10.1038/npp.2012.20
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2012.20
Keywords
This article is cited by
-
Bidirectional Associations among Nicotine and Tobacco Smoke, NeuroHIV, and Antiretroviral Therapy
Journal of Neuroimmune Pharmacology (2020)
-
Tobacco smoking and dopaminergic function in humans: a meta-analysis of molecular imaging studies
Psychopharmacology (2019)
-
Combination therapy of varenicline with nicotine replacement therapy is better than varenicline alone: a systematic review and meta-analysis of randomized controlled trials
BMC Public Health (2015)
-
Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals
Psychopharmacology (2014)