Abstract
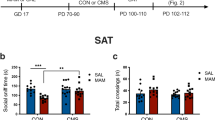
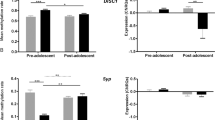
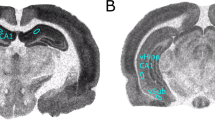
Schizophrenia is believed to arise from an interaction of genetic predisposition and adverse environmental factors, with stress being a primary variable. We propose that alleviating anxiety produced in response to stress during a sensitive developmental period may circumvent the dopamine (DA) system alterations that may correspond to psychosis in adults. This was tested in a developmental rat model of schizophrenia based on prenatal administration of the mitotoxin methyl azoxymethanol acetate (MAM). MAM administration leads to a hyperdopaminergic state consisting of an increase in the number of DA neurons firing spontaneously, which correlates with an increased behavioral response to amphetamine. MAM-treated rats exhibited a heightened level of anxiety during adolescence. Peripubertal administration of the antianxiety agent diazepam was found to prevent the increase in DA neuron activity and blunt the behavioral hyperresponsivity to amphetamine in these rats. These data suggest that the pathophysiological factors leading to the onset of psychosis in early adulthood may be circumvented by controlling the response to stress during the peripubertal period.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barretta S, Munno DW, Benes FM (2001). Amygdalar activation alters the hippocampal GABA system: ‘partial’ modelling for postmortem changes in schizophrenia. J Comp Neurol 431: 129–138.
Benes FM (1999). Evidence for altered trisynaptic circuitry in schizophrenic1 hippocampus. Biol Psychiatry 46: 589–599.
Cornblatt BA, Lencz T, Smith CW, Olsen R, Auther AM, Nakayama E et al (2007). Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J Clin Psychiatry 68: 546–557.
Conrad CD, Magarifios AM, LeDoux JE, McEwen BS (1999). Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113: 902–913.
Corcoran C, Walker EF, Huot R, Mittal V, Tessner K, Kestler L et al (2003). The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 29: 671–692.
Corcoran CM, Smith C, McLaughlin D, Auther A, Malaspina D, Cornblatt B (2012). HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr Res 135: 170–174.
Devylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, Corcoran CM (2013). Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychol Med 43: 259–268.
Ferguson SA, Boctor SY (2009). Use of food wafers for multiple daily oral treatments in young rats. J Am Assoc Lab Anim Sci 96: 292–296.
Flagstad P, Mørk A, Glenthøj BY, van Beek J, Michael-Titus AT, Didriksen M (2004). Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology 29: 2052–2064.
Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B et al (2005). Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry 58: 417–423.
Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA (2011). A novel α5GABAAR-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology 36: 1903–1911.
Goldstein JM, Seidman LJ, Makris N, Ahern T, O’Brien LM, Caviness VS et al (2007). Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry 61: 935–945.
Grace AA, Bunney BS (1983). Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience 10: 301–315.
Grace AA, Moore H, O'Donnell P (1998). The modulation of corticoaccumbens transmission by limbic afferents and dopamine: a model for the pathophysiology of schizophrenia. Adv Pharmacol 42: 721–724.
Goto Y, Grace AA (2006). Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biol Psychiatry 60: 1259–1267.
Habets P, Collip D, Myin-Germeys I, Gronenschild E, van Bronswijk S, Hofman P et al (2012). Pituitary volume, stress reactivity and genetic risk for psychotic disorder. Psychol Med 42: 1523–1533.
Hill M, Crumlish N, Clarke M, Whitty P, Owens E, Renwick L et al (2012). Prospective relationship of duration of untreated psychosis to psychopathology and functional outcome over 12 years. Schizophr Res 141: 215–221.
Holtzman CW, Shapiro DI, Trotman HD, Walker EF (2012). Stress and the prodromal phase of psychosis. Curr Pharm Des 18: 527–553.
Howes RD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR et al (2011). Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry 168: 1311–1317.
Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R (1999). Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 46: 56–72.
Lewis DA, Curley AA, Glausier JR, Volk DW (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35: 57–67.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO et al (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353: 1209–1223.
Lodge DJ, Grace AA (2007). Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27: 11424–11430.
Lodge DJ, Behrens MM, Grace AA (2009). A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci 29: 2344–2354.
Lodge DJ, Grace AA (2009). Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res 204: 306–312.
Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV et al (1998). Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1: 69–73.
Magarinos AM, McEwen BS (1995). Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of clucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69: 89–98.
Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A et al (1999). SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry 46: 89–93.
Maynard TM, Sikich L, Lieberman JA, LaMantia A-S (2001). Neural development, cell-cell signaling, and the ‘two-hit’ hypothesis of schizophrenia. Schizophr Bull 27: 457–476.
Medoff DR, Holcomb HH, Lahti AC, Tamminga CA (2001). Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus 11: 543–550.
Miller TJ, McGlashan TH, Rosen JL, Cadenhead KS, Ventura J, McFarlane W et al (2003). Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 29: 703–715.
Molina V, Reig S, Pascau J, Sanz J, Sarramea F, Gispert JD et al (2003). Anatomical and functional cerebral variables associated with basal symptoms but not risperidone response in minimally treated schizophrenia. Psychiatry Res 124: 163–175.
Mondelli V, Pariante CM, Navari S, Aas M, D'Albenzio A, Di Forti M et al (2010). Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr Res 119: 75–78.
Mondelli V, Cattaneo A, Murri MB, Di Forti M, Handley R, Hepgul N et al (2011). Stress and Inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis. J Clin Psychiatry 72: 1677–1684.
Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA (2006). A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry 60: 253–264.
Nelson MD, Saykin AJ, Flashman LA, Riordan HJ (1998). Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging. Arch Gen Psychiatry 55: 433–440.
Owens DG, Miller P, Lawrie SM, Johnstone EC (2005). Pathogenesis of schizophrenia: a psychopathological perspective. Br J Psychiatry 186: 386–393.
Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT (2006). Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci 23: 279–284.
Phillips LJ, Yung AR, Yuen HP, Pantelis C, McGorry PD (2002). Prediction and prevention of transition to psychosis in young people at incipient risk for schizophrenia. Am J Med Genet 114: 929–937.
Phillips LJ, Francey SM, Edwards J, McMurray N (2007). Stress and psychosis: Towards the development of new models of investigation. Clin Psychol Rev 27: 307–317.
Piontkewitz Y, Arad M, Weiner I (2011). Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull 37: 1257–1269.
Piontkewitz Y, Bernstein HG, Dobrowolny H, Bogerts B, Weiner I, Keilhoff G (2012). Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav Immun 26: 353–363.
Rosenkranz JA, Grace AA (2001). Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci 21: 4090–4103.
Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D et al (2009). Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 66: 938–946.
Stone JM, Howes OD, Egerton A, Kambeitz J, Allen P, Lythgoe DJ et al (2010). Altered relationship between hippocampal glutamate levels and striatal dopamine function in subjects at ultra high risk of psychosis. Biol Psychiatry 68: 599–602.
Straub RE, Weinberger DR (2006). Schizophrenia genes - famine to feast. Biol Psychiatry 60: 81–83.
Sullivan PF, Kendler KS, Neale MC (2003). Schizophrenia as a complex trait: eveidence from a meta-analysis of twin studies. Arch Gen Psychiatry 60: 1187–1192.
Thompson JL, Pogue-Geile MF, Grace AA (2004). Developmental pathology, dopamine, and stress: a model of the age of onset of schizophrenia symptoms. Schizophr Bull 30: 875–880.
Walker EF, Diforio D (1997). Schizophrenia: a neural diathesis-stress model. Psychol Rev 104: 667–685.
Walker E, Mittal V, Tessner K (2008). Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol 4: 189–216.
Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT (2010). Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnorm Psychol 119: 401–408.
Ungless MA, Grace AA (2012). Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35: 422–430.
Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'olio M et al (2005). Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry 39: 964–971.
Zhang ZJ, Reynolds GP (2002). A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 55: 1–10.
Acknowledgements
This work was supported by the US Public Health Service Grants MH57440 (AAG). We thank Niki MacMurdo for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Over the past 3 years, AAG has received compensation from Johnson & Johnson, Lundbeck, Pfizer, GSK, Puretech Ventures, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, and Asubio. YD declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Du, Y., Grace, A. Peripubertal Diazepam Administration Prevents the Emergence of Dopamine System Hyperresponsivity in the MAM Developmental Disruption Model of Schizophrenia. Neuropsychopharmacol 38, 1881–1888 (2013). https://doi.org/10.1038/npp.2013.101
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.101
Keywords
This article is cited by
-
Aberrant Hippocampal Development in Early-onset Mental Disorders and Promising Interventions: Evidence from a Translational Study
Neuroscience Bulletin (2023)
-
GABAA and NMDA receptor density alterations and their behavioral correlates in the gestational methylazoxymethanol acetate model for schizophrenia
Neuropsychopharmacology (2022)
-
Hippocampal circuit dysfunction in psychosis
Translational Psychiatry (2022)
-
Adult stress exposure blunts dopamine system hyperresponsivity in a neurodevelopmental rodent model of schizophrenia
Schizophrenia (2022)
-
Reduced cortical GABA and glutamate in high schizotypy
Psychopharmacology (2021)