Abstract
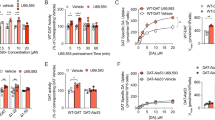
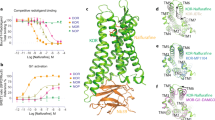
Kappa-opioid receptor (KOR) agonists have dysphoric properties in humans and are aversive in rodents. This has been attributed to the activation of KORs within the mesolimbic dopamine (DA) system. However, the role of DA in KOR-mediated aversion and stress remains divisive as recent studies have suggested that activation of KORs on serotonergic neurons may be sufficient to mediate aversive behaviors. To address this question, we used conditional knock-out (KO) mice with KORs deleted on DA neurons (DATCre/wt/KORloxp/loxp, or DATCre-KOR KO). In agreement with previous findings, control mice (DATCre/wt/KORwt/wt or WT) showed conditioned place aversion (CPA) to the systemically administered KOR agonist U69,593. In contrast, DATCre-KOR KO mice did not exhibit CPA with this same agonist. In addition, in vivo microdialysis showed that systemic U69,593 decreased overflow of DA in the nucleus accumbens (NAc) in WT mice, but had no effect in DATCre-KOR KO mice. Intra- ventral tegmental area (VTA) delivery of KORs using an adeno-associated viral gene construct, resulted in phenotypic rescue of the KOR-mediated NAc DA response and aversive behavior in DATCre-KOR KO animals. These results provide evidence that KORs on VTA DA neurons are necessary to mediate KOR-mediated aversive behavior. Therefore, our data, along with recent findings, suggest that the neuronal mechanisms of KOR-mediated aversive behavior may include both dopaminergic and serotonergic components.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ et al (2006). Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus. Genesis 44: 383–390.
Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993a). Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264: 489–495.
Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993b). Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264: 489–495.
Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ et al (2011). Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci 14: 1033–1038.
Carlezon WA Jr, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS et al (2006). Depressive-like effects of the {kappa}-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316: 440–447.
Carlezon WA Jr, Beguin C, Knoll AT, Cohen BM (2009). Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther 123: 334–343.
Carroll FI, Carlezon WA Jr (2013). Development of kappa opioid receptor antagonists. J Med Chem 56: 2178–2195.
Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS (2005). Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci 25: 5029–5037.
Chefer VI, Denoroy L, Zapata A, Shippenberg TS (2009). Mu opioid receptor modulation of somatodendritic dopamine overflow: GABAergic and glutamatergic mechanisms. Eur J Neurosci 30: 272–278.
Chefer VI, Kieffer BL, Shippenberg TS (2004). Contrasting effects of mu opioid receptor and delta opioid receptor deletion upon the behavioral and neurochemical effects of cocaine. Neuroscience 127: 497–503.
Chefer VI, Zapata A, Shippenberg TS, Bungay PM (2006). Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods 155: 187–193.
Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N (2012). Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482: 85–88.
Cunningham CL, Gremel CM, Groblewski PA (2006). Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1: 1662–1670.
Di Salvio M., Di Giovannantonio LG, Acampora D, Prosperi R, Omodei D, Prakash N et al (2010). Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci 13: 1481–1488.
Di SM, Di Giovannantonio LG, Acampora D, Prosperi R, Omodei D, Prakash N et al (2010). Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci 13: 1481–1488.
Diaz-Ruiz O, Zapata A, Shan L, Zhang Y, Tomac AC, Malik N et al (2009). Selective deletion of PTEN in dopamine neurons leads to trophic effects and adaptation of striatal medium spiny projecting neurons. PLoS One 4: e7027.
Diaz-Ruiz O, Zhang Y, Shan L, Malik N, Hoffman AF, Ladenheim B et al (2012). Attenuated response to methamphetamine sensitization and deficits in motor learning and memory after selective deletion of beta-catenin in dopamine neurons. Learn Mem 19: 341–350.
Heijna MH, Padt M, Hogenboom F, Portoghese PS, Mulder AH, Schoffelmeer AN (1990). Opioid receptor-mediated inhibition of dopamine and acetylcholine release from slices of rat nucleus accumbens, olfactory tubercle and frontal cortex. Eur J Pharmacol 181: 267–278.
Hjelmstad GO, Fields HL (2001). Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol 85: 1153–1158.
Hjelmstad GO, Fields HL (2003). Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol 89: 2389–2395.
Horvitz JC (2000). Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 96: 651–656.
Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG et al (2011). Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry 70: 425–433.
Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D et al (2009). Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA 106: 19168–19173.
Lubow RE (1973). Latent inhibition. Psychol Bull 79: 398–407.
Luo Y, Good CH, Diaz-Ruiz O, Zhang Y, Hoffman AF, Shan L et al (2010). NMDA receptors on non-dopaminergic neurons in the VTA support cocaine sensitization. PLoS One 5: e12141.
Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL (2006). {kappa} opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA 103: 2938–2942.
Mucha RF, Herz A (1985). Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 86: 274–280.
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates. Academic Press, 350 p.
Pfeiffer A, Brantl V, Herz A, Emrich HM (1986). Psychotomimesis mediated by kappa opiate receptors. Science 233: 774–776.
Rawls SM, McGinty JF (1998). Kappa receptor activation attenuates L-trans-pyrrolidine-2,4-dicarboxylic acid-evoked glutamate levels in the striatum. J Neurochem 70: 626–634.
Shippenberg TS, Bals-Kubik R, Herz A (1993). Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther 265: 53–59.
Shippenberg TS, Elmer GI (1998). The neurobiology of opiate reinforcement. Crit Rev Neurobiol 12: 267–303.
Shippenberg TS, Herz A, Spanagel R, Bals-Kubik R, Stein C (1992). Conditioning of opioid reinforcement: neuroanatomical and neurochemical substrates. Ann N Y Acad Sci 654: 347–356.
Spanagel R, Almeida OF, Bartl C, Shippenberg TS (1994). Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl ) 115: 121–127.
Spanagel R, Herz A, Shippenberg TS (1992). Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA 89: 2046–2050.
Svingos AL, Chavkin C, Colago EE, Pickel VM (2001). Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse 42: 185–192.
Svingos AL, Colago EE, Pickel VM (1999). Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci 19: 1804–1813.
Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Backman CM et al (2013). Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38: 1770–1779.
Thompson AC, Zapata A, Justice JB, Vaughan RA, Sharpe LG, Shippenberg TS (2000). kappa-Opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci 20: 9333–9340.
Van't Veer A, Bechtholt AJ, Onvani S, Potter D, Wang Y, Liu-Chen LY et al (2013). Ablation of kappa-opioid receptors from brain dopamine neurons has anxiolytic-like effects and enhances cocaine-induced plasticity. Neuropsychopharmacology 38: 1585–1597.
Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ (2005). Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 179: 551–558.
Zhang Y, Granholm AC, Huh K, Shan L, Diaz-Ruiz O, Malik N et al (2012). PTEN deletion enhances survival, neurite outgrowth and function of dopamine neuron grafts to MitoPark mice. Brain 135: 2736–2749.
Acknowledgements
This work was supported by the National Institute on Drug Abuse Intramural Research Program. We thank Dr Carl Lupica for his helpful editorial comments and suggestions; and Dr Jennifer Whistler for providing KORloxp mice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
V.C. and T.S.S. designed the research; C.M.B. created conditional knock-outs; V.C., E.D.G., and C.M.B. performed the research; V.C. and E.D.G. analyzed the data; V.C., E.D.G., and C.M.B. wrote the paper.
Rights and permissions
About this article
Cite this article
Chefer, V., Bäckman, C., Gigante, E. et al. Kappa Opioid Receptors on Dopaminergic Neurons Are Necessary for Kappa-Mediated Place Aversion. Neuropsychopharmacol 38, 2623–2631 (2013). https://doi.org/10.1038/npp.2013.171
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.171
Keywords
This article is cited by
-
Optogenetic stimulation of lateral hypothalamic orexin/dynorphin inputs in the ventral tegmental area potentiates mesolimbic dopamine neurotransmission and promotes reward-seeking behaviours
Neuropsychopharmacology (2022)
-
Kappa opioid receptor and dynorphin signaling in the central amygdala regulates alcohol intake
Molecular Psychiatry (2021)
-
The kappa opioid receptor agonist U50,488H did not affect brain-stimulation reward while it elicited conditioned place aversion in mice
BMC Research Notes (2020)
-
Individual variation in the attribution of incentive salience to social cues
Scientific Reports (2020)
-
Kappa opioid agonists reduce oxycodone self-administration in male rhesus monkeys
Psychopharmacology (2020)