Abstract
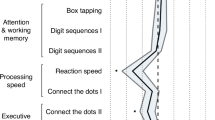
Tamoxifen (TMX) is a selective estrogen receptor modulator that is used as an estrogen receptor antagonist for the treatment and prevention of breast cancer. Whether TMX has antagonist activities in the human brain is less clear and its effects on cognitive function have not been experimentally explored. This study examined how TMX affected cognitive performance in older women using a model of anticholinergic drug-induced cognitive dysfunction. Twenty-one postmenopausal women were administered 20 mg of oral TMX or placebo for 3 months. Participants then took part in five drug challenges using the anticholinergic antinicotinic agent mecamylamine (MECA) and antimuscarinic agent scopolamine (SCOP) and were tested on a comprehensive battery including tasks of attention and psychomotor function, verbal episodic memory, and spatial navigation. After a 3-month placebo washout, participants were then crossed over to the alternate treatment and repeated the drug challenges after 3 months. Compared with placebo treatment, TMX significantly attenuated the impairment from cholinergic blockade on tasks of verbal episodic memory and spatial navigation, but effects on attentional/psychomotor tasks were more variable. Analysis by APOE genotype showed that APO ɛ4+ women showed a greater beneficial effect of TMX on reversing the cholinergic impairment than APO ɛ4− women on most tasks. This study provides evidence that TMX may act as an estrogen-like agonist to enhance cholinergic system activity and hippocampally mediated learning.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B et al (2003). The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-Oncology 12: 612–619.
Astur RS, Ortiz ML, Sutherland RJ (1998). A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res 93: 185–190.
Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ (2002). Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res 132: 77–84.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961). An inventory for measuring depression. Arch Gen Psychiatr 4: 53–63.
Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M (2001). Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA 98: 13391–13395.
Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM (2007). Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72: 381–405.
Breckenridge LM, Bruns GL, Todd BL, Feuerstein M (2012). Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psycho-Oncology 21: 43–53.
Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE (2003). The role of APOE-ɛ4 in longitudinal cognitive decline: MacArthur studies of successful aging. Neurology 60: 1077–1081.
Breuer B, Anderson R (2000). The relationship of tamoxifen with dementia, depression, and dependence in activities of daily living in elderly nursing home residents. Women Health 31: 71–86.
Brinton RD (2002). Selective estrogen receptor modulators (SERM) for the brain: recent advances and remaining challenges for developing a NeuroSERM (TM). Drug Develop Res 56: 380–392.
Buschke H (1973). Selective reminding for analysis of memory and learning. J Verbal Learn Verbal Behav 12: 543–550.
Chen D, Chun-Fu W, Shi B, Xu Y (2002). Tamoxifen and toremifene impair retrieval, but not acquisition, of spatial information processing in mice. Pharmacol Biochem Behav 72: 417–421.
Ciriza I, Carrero P, Azcoitia I, Lundeen SG, Garcia-Segura LM (2004). Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: differences with the effect of estradiol. J Neurobiol 61: 209–221.
Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P et al (2010). Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS). J Steroid Biochem Mol Biol 118: 304–310.
Collaborative GoHFiBC (1997). Breast cancer and hormone replacement-therapy: collaborative reanalysis of data from 51 epidemiologic studies of 52,705 women with breast cancers and 108,411 women without breast cancer. Lancet 350: 1047–1059.
Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T (2001a). Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Rev 37: 153–161.
Cyr M, Morissette M, Landry M, Di Paolo T (2001b). Estrogenic activity of tamoxifen and raloxifene on rat brain AMPA receptors. Neuroreport 12: 535–539.
Cyr M, Thibault C, Morissette M, Landry M, Di Paolo T (2001c). Estrogen-like activity of tamoxifen and raloxifene on NMDA receptor binding and expression of its subunits in rat brain. Neuropsychopharmacology 25: 242–257.
Daniel JM, Hulst JL, Lee CD (2005). Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience 132: 57–64.
Dhandapani KM, Brann DW (2003). Neuroprotective effects of estrogen and tamoxifen in vitro. Endocrine 21: 59–66.
Dohanich GP, Fader AJ, Javorsky DJ (1994). Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci 108: 988–992.
Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P (2008). Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Hormone Behav 53: 159–169.
Dumas JA, Hancur-Bucci C, Naylor M, Sites C, Newhouse PA (2006). Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal post-menopausal women. Neuropsychopharmacology 31: 2065–2078.
Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA (2012). Estradiol reversal of anticholinergic-related brain activation in postmenopausal women. Neuroimage 60: 1394–1403.
Dumas JA, Newhouse PA (2011). The cholinergic hypothesis of cognitive aging revisited again: Cholinergic functional compensation. Pharmacol Biochem Behav 99: 254–261.
Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ (2004). Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage 21: 364–371.
Ernst T, Chang L, Cooray D, Salvador C, Jovicich J, Walot I et al (2002). The effects of tamoxifen and estrogen on brain metabolism in elderly women. J Natl Cancer Inst 94: 592–597.
Esmaeili B, Basseda Z, Gholizadeh S, Javadi Paydar M, Dehpour AR (2009). Tamoxifen disrupts consolidation and retrieval of morphine-associated contextual memory in male mice: interaction with estradiol. Psychopharmacology (Berl) 204: 191–201.
Ewertz M, Mellemkjaer L, Poulsen AH, Friis S, Sorensen HT, Pedersen L et al (2005). Hormone use for menopausal symptoms and risk of breast cancer. A Danish cohort study. Br J Cancer 92: 1293–1297.
Fader AJ, Johnson PEM, Dohanich GP (1999). Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav 62: 711–717.
Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips KA (2005). The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain Cogn 59: 60–70.
Fester L, Ribeiro-Gouveia V, Prange-Kiel J, von Schassen C, Bottner M, Jarry H et al (2006). Proliferation and apoptosis of hippocampal granule cells require local oestrogen synthesis. J Neurochem 97: 1136–1144.
First MB, R.L. S, Gibbon M, Williams JBW (2001) Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition, SCID-I/P, 2/2001 edn American Psychiatric Press Inc: Washington, DC, USA.
Gibbs RB (1998). Impairment of basal forebrain cholinergic neurons associated with aging and long-term loss of ovarian function. Exp Neurol 151: 289–302.
Gibbs RB (1999). Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormone Behav 36: 222–233.
Gibbs RB (2000). Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience 101: 931–938.
Gibbs RB (2001). Estrogen enhancement of learning requires intact basal forebrain cholinergic neutrons. Soci Neurosci Abstr 31: 312.
Gibbs RB (2010). Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocrine Rev 31: 224–253.
Gonzalez-Burgos I, Rivera-Cervantes MC, Velazquez-Zamora DA, Feria-Velasco A, Garcia-Segura LM (2012). Selective estrogen receptor modulators regulate dendritic spine plasticity in the hippocampus of male rats. Neural Plast 2012: 309494.
Hammond R, Gibbs RB (2011). GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res 1379: 53–60.
Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WGM, Lou W et al (2006). Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. Jf Neurosci 26: 2571–2578.
Hazell GGJ, Yao ST, Roper JA, Prossnitz ER, O'Carroll A-M, Lolait SJ (2009). Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 202: 223–236.
Hindmarch I (1984). Psychological performance models as indicators of the effects of hypnotic drugs on sleep. Psychopharmacology S1: 58–68.
Hoddes ElZ V, Smythe H, Phillips R, Dement W (1973). Quantification of sleepiness: a new approach. Psychophysiology 10: 431–436.
Hulley S, Grady D (2004). The WHI estrogen-alone trial—do things look any better? J Am Med Assocn 291: 1769–1771.
Ignatov A, Ignatov T, Roessner A, Costa S, Kalinski T (2010). Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Res Treat 123: 87–96.
Kang JH, Grodstein F (2012). Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiol Aging 33: 1129–1137.
Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ et al (2004). Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors α and β. Mol Biol Cell 15: 1262–1272.
Kristensen B, Ejlertsen B, Dalgaard P, Larsen L, Holmegaard SN, Transbol I et al (1994). Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J Clin Oncol 12: 992–997.
Lagunas N, Calmarza-Font I, Grassi D, Garcia-Segura LM (2011). Estrogen receptor ligands counteract cognitive deficits caused by androgen deprivation in male rats. Hormone Behav 59: 581–584.
Legault C, Maki PM, Resnick SM, Coker L, Hogan P, Bevers TB et al (2009). Effects of tamoxifen and raloxifene on memory and other cognitive abilities: cognition in the study of tamoxifen and raloxifene. J Clin Oncol 27: 5144–5152.
Li X, Takahashi M, Kushida K, Koyama S, Hoshino H, Kawana K et al (1996). The effect of tamoxifen on bone metabolism and skeletal growth is different in ovariectomized and intact rats. Calc Tissue Int 59: 271–276.
Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R et al (2008). Activation of estrogen receptor-[beta] regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci 11: 334–343.
Maggiolini M, Picard D (2010). The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol 204: 105–114.
Maki PM (2005). A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann NY Acad Sci 1052: 182–197.
Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J et al (2007). Estrogen therapy and coronary-artery calcification. N Engl J Med 356: 2591–2602.
McInerney EM, Weis KE, Sun J, Mosselman S, Katzenellenbogen BS (1998). Transcription activation by the human estrogen receptor subtypeβ (ERβ) studied with ERβ and ERα receptor chimeras. Endocrinology 139: 4513–4522.
McMillan PJ, LeMaster AM, Dorsa DM (2002). Tamoxifen enhances choline acetyltransferase mRNA expression in rat basal forebrain cholinergic neurons. Mol Brain Res 103: 140–145.
McNair DM, Lorr M, Droppleman LF (1971) Profile of Mood States. Educational and Industrial Testing Service: San Diego, CA, USA.
Moreira PI, Custódio JB, Oliveira CR, Santos MS (2005). Brain mitochondrial injury induced by oxidative stress-related events is prevented by tamoxifen. Neuropharmacology 48: 435–447.
Mortensen EL, Høgh P (2001). A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology 57: 89–95.
Newhouse P, Newhouse C, Astur RS (2007). Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res 183: 1–7.
Newhouse PA, Potter A, Corwin J, Lenox R (1994). Age-related effects of the nicotinic antagonist mecamylamine on cognition and behavior. Neuropsychopharmacology 10: 93–107.
Norbury R, Travis MJ, Erlandsson K, Waddington W, Ell PJ, Murphy DGM (2007). Estrogen therapy and brain muscarinic receptor density in healthy females: A SPET study. Hormone Behav 51: 249–257.
Obata T, Kubota S (2001). Protective effect of tamoxifen on 1-methyl-4-phenylpyridine-induced hydroxyl radical generation in the rat striatum. Neurosci Lett 308: 87–90.
Osborne CK (1998). Tamoxifen in the treatment of breast cancer. N Engl J Med 339: 1609–1618.
Overall J, Gorham D (1993). The brief psychiatric rating scale. Psycholo Rep 10: 799–812.
Paganini-Hill A, Clark LJ (2000). Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat 64: 165–176.
Palmer JL, Trotter T, Joy AA, Carlson LE (2008). Cognitive effects of Tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. J Cancer Surviv 2: 275–282.
Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, Lahiri D et al (1995). Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci USA 92: 12260–12264.
Powles TJ (2012). Extended adjuvant tamoxifen for breast cancer—a new era? Lancet 381: 782–783.
Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S (1996). Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol 14: 78–84.
Rossouw JE, Anderson GL, Investigators WGftWsHI (2002). Risks and benefits of estrogen plus progestin in healthy postmenpausal women. J Am Med Assoc 288: 321–331.
Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM et al (2007). Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. J Am Med Assoc 297: 1465–1477.
Sharma K, Mehra RD (2008). Long-term administration of estrogen or tamoxifen to ovariectomized rats affords neuroprotection to hippocampal neurons by modulating the expression of Bcl-2 and Bax. Brain Res 1204: 1–15.
Sherwin BB (2007). The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature. J Neuroendocrinol 19: 88–81.
Shughrue PJ, Scrimo PJ, Merchenthaler I (2000). Estrogen binding and estrogen receptor characterization (ERα and ERβ) in the cholinergic neurons of the rat basal forebrain.. Neuroscience 96: 41–49.
Silva I, LEAM Mello, Freymuller E, Haidar MA, Baracat EC (2000). Estrogen, progestogen and tamoxifen increase synaptic density of the hippocampus of ovariectomized rats. Neurosci Lett 291: 183–186.
Singh M, Meyer EM, Millard WJ, Simpkins JW (1994). Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 644: 305–312.
Tanabe F, Miyasaka N, Kubota T, Aso T (2004). Estrogen and progesterone improve scopolamine-induced impairment of spatial memory. J Med Dental Sci 51: 89–98.
Tinkler GP, Voytko ML (2005). Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuro-Psychopharmacol Biol Psychiatr 29: 423–431.
Vehmanen L, Elomaa I, Blomqvist C, Saarto T (2006). Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol 24: 675–680.
Velazquez-Zamora DA, Garcia-Segura LM, Gonzalez-Burgos I (2012). Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Hormones Behav 61: 512–517.
Wakade C, Khan MM, De Sevilla LM, Zhang Q-G, Mahesh VB, Brann DW (2008). Tamoxifen neuroprotection in cerebral ischemia involves attenuation of kinase activation and superoxide production and potentiation of mitochondrial superoxide dismutase. Endocrinology 149: 367–379.
Wang JM, Irwin RW, Brinton RD (2006). Activation of estrogen receptor α increases and estrogen receptor β decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci USA 103: 16983–16988.
Ward HW (1973). Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels. Br Med J 1: 13–14.
Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A et al (2003). Effect of Estrogen Plus Progestin on Stroke in Postmenopausal Women. J Am Med Assoc 289: 2673–2684.
Weber M, Mapstone M (2009). Memory complaints and memory performance in the menopausal transition. Menopause 16: 1–7.
Woolley CS, McEwen BS (1993). Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Compar Neurol 336: 293–306.
Wu X, Glinn MA, Ostrowski NL, Su Y, Ni B, Cole HW et al (1999). Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res 847: 98–104.
Yaffe KM, Haan MD, Byers AM, Tangen CD, Kuller LMDD (2000). Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology 54: 1949–1954.
Yamamoto H, Kitawaki J, Kikuchi N, Okubo T, Iwasa K, Kawata M et al (2007). Effects of estrogens on cholinergic neurons in the rat basal nucleus. J Steroid Biochem Mol Biol 107: 70–79.
Yankova M, Hart SA, Woolley CS (2001). Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci USA 98: 2941–3624.
Acknowledgements
We wish to acknowledge the invaluable contributions of Dr Emily Coderre, Dr Jessica Salerno, Christy Edgren, the nursing staff of the Clinical Research Center at the University of Vermont, and our research volunteers for their dedication to clinical research. This work was supported by NIA R01 AG021476, NIA K01 030380, and NCRR M01-00109.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newhouse, P., Albert, K., Astur, R. et al. Tamoxifen Improves Cholinergically Modulated Cognitive Performance in Postmenopausal Women. Neuropsychopharmacol 38, 2632–2643 (2013). https://doi.org/10.1038/npp.2013.172
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.172
Keywords
This article is cited by
-
Nicotine sensitization (part 1): estradiol or tamoxifen is required during the induction phase and not the expression phase to enable locomotor sensitization to nicotine in female rats
Psychopharmacology (2021)
-
The Role of Estrogen in Brain and Cognitive Aging
Neurotherapeutics (2019)
-
A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy
Journal of Cancer Survivorship (2016)
-
The Impact of Endocrine Therapy on Cognitive Functions of Breast Cancer Patients: A Systematic Review
Clinical Drug Investigation (2016)