Abstract
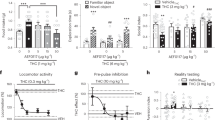
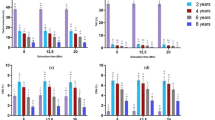
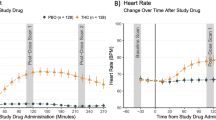
Few individuals seeking treatment for marijuana use achieve sustained abstinence. The cannabinoid receptor agonist, Δ9-tetrahydrocannabinol (THC; dronabinol), decreases marijuana withdrawal symptoms, yet does not decrease marijuana use in the laboratory or clinic. Dronabinol has poor bioavailability, which may contribute to its poor efficacy. The FDA-approved synthetic analog of THC, nabilone, has higher bioavailability and clearer dose-linearity than dronabinol. This study tested whether nabilone administration would decrease marijuana withdrawal symptoms and a laboratory measure of marijuana relapse relative to placebo. Daily, nontreatment-seeking marijuana smokers (8 men and 3 women), who reported smoking 8.3±3.1 marijuana cigarettes/day completed this within-subject study comprising three, 8-day inpatient phases; each phase tested a different nabilone dose (0, 6, 8 mg/day, administered in counter-balanced order on days 2–8). On the first inpatient day, participants took placebo capsules and smoked active marijuana (5.6% THC) at six timepoints. For the next 3 days, they had the opportunity to self-administer placebo marijuana (0.0% THC; withdrawal), followed by 4 days in which active marijuana was available for self-administration (5.6% THC; relapse). Both nabilone dose conditions decreased marijuana relapse and reversed withdrawal-related irritability and disruptions in sleep and food intake (p<0.05). Nabilone (8 mg/day) modestly worsened psychomotor task performance. Neither dose condition increased ratings of capsule ‘liking’ or desire to take the capsules relative to placebo. Thus, nabilone maintenance produced a robust attenuation of marijuana withdrawal symptoms and a laboratory measure of relapse even with once per day dosing. These data support testing of nabilone for patients seeking marijuana treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J et al (2012). Quantifying the clinical significance of cannabis withdrawal. PLoS One 7: e44864.
Bedi G, Cooper Z, Haney M (2012). Subjective, cognitive, and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addiction Biol e-pub ahead of print 19 January 2012. doi: 10.1111/j.1369-1600.2011.00427.x.
Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD et al (2010). Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology (Berl) 212: 675–686.
Ben Amar M (2006). Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol 105: 1–25.
Budney AJ, Moore BA, Rocha HL, Higgins ST (2006). Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol 74: 307–316.
Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B (2007). Oral delta-9 tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend 86: 22–29.
Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM (2012). Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction 107: 1650–1659.
Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL (2008). The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend 96: 1–15.
Compton W, Thomas Y, Stinson FS, Grant B (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States—results from the National Epidemiologic Survey of Alcohol and Related conditions. Arch Gen Psychiatry 64: 566–576.
Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M (2012). A controlled human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addiction Biol e-pub ahead of print 28 June 2012. doi: 10.1111/j.1369-1600.2012.00461.x.
Copeland J, Swift W, Roffman R, Stephens R (2001). A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat 21: 55–64.
EMCDDA (2009). Annual Report on the State of the Drugs Problem in Europe www.emcdda.europa.eu/attachements.cfm/att_93236_EN_EMCDDA_AR2009_EN.pdf.
Evans SM, Foltin RW, Levin FR, Fischman MW (1995). Behavioral and subjective effects of DN-2327 (pazinaclone) and alprazolam in normal volunteers. Behav Pharmacol 6: 176–186.
Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD (1987). Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav 28: 459–464.
Foltin RW, Haney M, Comer SD, Fischman MW (1996). Effects of fenfluramine in food intake, mood, and performance of humans living in a residential laboratory. Physiol Behav 59: 295–305.
Fraser AD, Meatherall R (1989). Lack of interference by nabilone in the EMIT d.a.u. cannabinoid assay, Abbott TDx cannabinoid assay, and a sensitive TLC assay for delta 9-THC-carboxylic acid. J Anal Toxicol 13: 240.
Glass RM, Uhlenhuth EH, Hartel FW (1979). The effects of nabilone, a synthetic cannabinoid, on anxious human volunteers [proceedings]. Psychopharmacol Bull 15: 88–90.
Glass RM, Uhlenhuth EH, Hartel FW, Schuster CR, Fischman MW (1981). Single-dose study of nabilone in anxious volunteers. J Clin Pharmacol 21: 383S–396S.
Hall W (2006). The mental health risks of adolescent cannabis use. PLoS Medicine 3: 159–162.
Haney M (2009). Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol 14: 9–21.
Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD et al (2012). Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biological Psychiatry 73: 242–248.
Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD et al (2010). Effects of baclofen and mirtazapine on laboratory model of marijuana withdrawal and relapse. Psychopharmacol 211: 233–244.
Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW (2008). Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacol 197: 157–168.
Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C et al (2004). Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacol 29: 158–170.
Haney M, Spealman R (2008). Controversies in translational research: drug self-administration. Psychopharmacol 199: 403–419.
Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW (1999). Abstinence symptoms following smoked marijuana in humans. Psychopharmacol 141: 395–404.
Hart C, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW (2002). Comparison of smoked marijuana and oral delta(9)-tetrahydrocannabinol in humans. Psychopharmacol 164: 407–415.
Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE (2010) Monitoring the future national survey results on drug use, 1975-2008 Vol I. Secondary School Students. National Institute on Drug Abuse: Bethesda, MD, USA).
Kadden RM, Litt MD, Kabela-Cormier E, Petry NM (2007). Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav 32: 1220–1236.
Kalliomäki J, Philipp A, Baxendale J, Annas P, Karlsten R, Segerdahl M (2012). Lack of effect of central nervous system-active doses of nabilone on capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol 39: 336–342.
Lemberger L, Rubin A, Wolen R, DeSante K, Rowe H, Forney R et al (1982). Pharmacokinetics, metabolism and drug-abuse potential of nabilone. Cancer Treat Rev 9 (Suppl B): 17–23.
Levin FR, Mariani JJ, Brooks DJ, Xie S, Murray KA (2010). Δ9-tetrahydrocannabivarin testing may not have the sensitivity to detect marijuana use among individuals ingesting dronabinol. Drug Alcohol Depend 1: 65–68.
Lile JA, Kelly TH, Hays LR (2010). Substitution profile of the cannabinoid agonist nabilone in human subjects discriminating δ9-tetrahydrocannabinol. Clin Neuropharmacol 33: 235–242.
Lile JA, Kelly TH, Hays LR (2011). Separate and combined effects of the cannabinoid agonists nabilone and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend. http://www.ncbi.nlm.nih.gov/pubmed?term=separate and combined effects of the cannabinoid aconists nabilone 116: 86–92.
Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF et al (2011). Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 115: 120–130.
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564.
McGilveray I (2005). Pharmacokinetics of cannabinoids. Pain Res Manag 10: 15A–22A.
Mendelson JH, Mello NK (1984). Reinforcing properties of oral delta 9-tetrahydrocannabinol, smoked marijuana, and nabilone: influence of previous marijuana use. Psychopharmacology (Berl) 83: 351–356.
MTPRG (2004). Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol 72: 455–466.
Rubin A, Lemberger L, Warrick P, Crabtree RE, Sullivan H, Rowe H et al (1977). Physiologic disposition of nabilone, a cannabinol derivative, in man. Clin Pharmacol Ther 22: 85–91.
Schierenbeck T, Riemann D, Berger M, Hornyak M (2008). Effect of illicit recreational drugs upon sleep: cocaine, ecstasy, and marijuana. Sleep Med Rev 12: 381–389.
Stephens RS, Roffman RA, Curtin L (2000). Extended versus brief treatment for marijuana use. J Consult Clin Psychol 68: 898–908.
Substance Abuse and Mental Health Services Administration (SAMHSA) (2012). Results from the 2011 National Survey on Drug Use and Health: Detailed Tables, www.samhsa.gov/data/NSDUH/2011SummNatFindDetTables/NSDUH-DetTabsPDFWHTML2011/2k11DetailedTabs/Web/PDFW/NSDUH-DetTabsCover2011.pdf.
Vandrey R, Umbricht A, Strain EC (2011). Increased blood pressure after abrupt cessation of daily cannabis use. J Addict Med 5: 16–20.
Wagner FA, Anthony JC (2002). Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am J Epidemiol 155: 918–925.
Ware MA, Arnaud-Trempe E (2010). The abuse potential of the synthetic cannabinoid nabilone. Addiction 105: 494–503.
Acknowledgements
The US National Institute on Drug Abuse (NIDA) supported this research (DA09236) and supplied the marijuana cigarettes. We are grateful to Laura Rolfe, Christina Hadzitheodorou, and Ashley Danies for their expert assistance in data collection and to Dr Adam Bisaga for medical supervision of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Haney’s research is funded by NIDA. She and Dr Foltin have received partial salary support for an investigator-initiated study from Astra-Zeneca. Dr Haney has also served as a consultant for GW Pharmaceuticals. Dr Comer has received partial salary support from investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals, Schering-Plough Corporation, Johnson & Johnson Pharmaceutical Research & Development, Endo Pharmaceuticals, and MediciNova. In addition, she and Dr Vosburg have received compensation from Grunenthal GmbH to conduct a meta-analysis of drug-induced subjective responses. Dr Comer has also served as a consultant to the following companies: Analgesic Solutions, BioDelivery Sciences International, Cephalon, Inflexxion, Innovative Science Solutions, Janssen, King, Neuromed, Pfizer, and Salix. Drs Cooper and Bedi declare no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Haney, M., Cooper, Z., Bedi, G. et al. Nabilone Decreases Marijuana Withdrawal and a Laboratory Measure of Marijuana Relapse. Neuropsychopharmacol 38, 1557–1565 (2013). https://doi.org/10.1038/npp.2013.54
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.54
Keywords
This article is cited by
-
Helpful or Harmful? The Therapeutic Potential of Medications with Varying Degrees of Abuse Liability in the Treatment of Substance Use Disorders
Current Addiction Reports (2022)
-
The emergency department care of the cannabis and synthetic cannabinoid patient: a narrative review
International Journal of Emergency Medicine (2021)
-
Cannabis use and cannabis use disorder
Nature Reviews Disease Primers (2021)
-
Pharmacological management of psychoactive substance withdrawal syndrome
Drugs & Therapy Perspectives (2021)
-
Evidence for the Endocannabinoid System as a Therapeutic Target in the Treatment of Cannabis Use Disorder
Current Addiction Reports (2020)