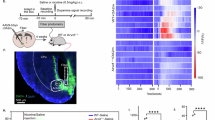
Abstract
The mesolimbic pathway comprising the ventral tegmental area (VTA) and projection terminals in the nucleus accumbens (NAc) has been identified as a critical neural system involved in processing both the rewarding and aversive behavioral effects of nicotine. Transmission through dopamine (DA) receptors functionally modulates these effects directly within the NAc. Nevertheless, the neuronal mechanisms within the NAc responsible for these bivalent behavioral effects are presently not known. Using an unbiased conditioned place preference procedure combined with in vivo neuronal recordings, we examined the effects of nicotine reward and aversion conditioning on intra-NAc neuronal sub-population activity patterns. We report that intra-VTA doses of nicotine that differentially produce rewarding or aversive behavioral effects produce opposite effects on sub-populations of fast-spiking interneurons (FSIs) or medium spiny neurons (MSNs) within the shell region of the NAc (NAshell). Thus, while the rewarding effects of intra-VTA nicotine were associated with inhibition of FSI and activation of MSNs, the aversive effects of nicotine produced the opposite pattern of NAshell neuronal population activity. Blockade of DA transmission with a broad-spectrum DA receptor antagonist, α-flupenthixol, strongly inhibited the spontaneous activity of NAshell FSIs, and reversed the conditioning properties of intra-VTA nicotine, switching nicotine-conditioned responses from aversive to rewarding. Remarkably, DA receptor blockade switched intra-NAshell neuronal population activity from an aversion to a reward pattern, concomitant with the observed switch in behavioral conditioning effects.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aosaki T, Kiuchi K, Kawaguchi Y (1998). Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. J Neurosci 18: 5180–5190.
Berger TW, Nisenbaum ES, Stricker EM, Zigmond MJ (1987). Evidence for two functionally distinct subpopulations of neurons within striatum and their differential sensitivity to dopamine. In: Chiodo LA, Freeman AS (eds.) Neurophysiology of Dopaminergic Systems. Lakeshore: Detroit, USA. pp 253–284.
Berke JD, Okatan M, Skurski J, Eichenbaum HB (2004). Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43: 883–896.
Bracci E, Centonze D, Bernardi G, Calabresi P (2002). Dopamine excites fast-spiking interneurons in the striatum. J Neurophysiol 87: 2190–2194.
Cadoni C, Muto T, Di Chiara G (2009). Nicotine differentially affects dopamine transmission in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. Neuropharmacology 57: 496–501.
Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF (2002). Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav 77: 683–687.
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006). Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology 184: 353–366.
Corrigall WA, Coen KM, Adamson KL (1994). Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653: 278–284.
Corrigall WA, Coen KM, Zhang J, Adamson KL (2001). GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology 158: 190–197.
Corrigall WA, Franklin KB, Coen KM, Clarke PB (1992). The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 107: 285–289.
Creese I, Burt DR, Snyder SH (1976). Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192: 481–483.
David V, Besson M, Changeux JP, Granon S, Cazala P (2006). Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology 50: 1030–1040.
English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Diesseroth K et al (2012). GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci 15: 123–130.
Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD (2010). Selective activation of striatal fast-spiking interneurons during choice execution. Neuron 67: 466–479.
Hu XT, Wachtel SR, Galloway MP, White FJ (1990). Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate-putamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulation. J Neurosci 10: 2318–2329.
Hu XT, White FJ (1997). Dopamine enhances glutamate-induced excitation of rat striatal neurons by cooperative activation of D1 and D2 class receptors. Neurosci Lett 224: 61–65.
Inglis WL, Dunbar JS, Winn P (1994). Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and responding for conditioned reinforcement stimulated by d-amphetamine. Neuroscience 62: 51–64.
Jorenby DE, Steinpreis RE, Sherman JE, Baker TB (1990). Aversion instead of preference learning indicated by nicotine place conditioning in rats. Psychopharmacology 101: 533–538.
Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H (2008). Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probalistic classical conditioning trials. J Neurosci 28: 11673–11684.
Kimura M, Rajkowski J, Evarts E (1984). Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci USA 81: 4998–5001.
Koos T, Tepper JM (1999). Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467–472.
Lansink CS, Goltstein M, Lankelma JV, Pennartz CMA (2010). Fast-spiking interneurons of the rat ventral striatum: temporal coordination of activity with principal cells and responsiveness to reward. Eur J Neurosci 32: 494–508.
Lança AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA (2000). The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience 96: 735–742.
Laviolette SR, Alexson TO, van der Kooy D (2002). Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J Neurosci 22: 8653–8660.
Laviolette SR, Lauzon NM, Bishop SF, Sun N, Tan H (2008). Dopamine signaling through D1-like versus D2-like receptors in the nucleus accumbens core versus shell differentially modulates nicotine reward sensitivity. J Neurosci 28: 8025–8033.
Laviolette SR, Nader K, van der Kooy D (2002). Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res 129: 17–29.
Laviolette SR, van der Kooy D (2004). The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5: 55–65.
Laviolette SR, van der Kooy D (2003). Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry 8: 50–59.
Ma YY, Henley SM, Toll J, Jentsch D, Evans CJ, Levine MS et al (2013). Drug-primed reinstatement of cocaine seeking in mice: increased excitability of medium-sized spiny neurons in the nucleus accumbens. ASN Neuro 5: 257–271.
Mansvelder HD, Keath JR, McGehee DS (2002). Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33: 905–919.
Mereu G, Yoon KW, Boi V, Gessa GL, Naes L, Westfall TC (1987). Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol 141: 395–399.
Morra JT, Glick SD, Cheer JF (2010). Neural encoding of psychomotor activation in the nucleus accumbens core, but not the shell, requires cannabinoid receptor signaling. J Neurosci 30: 5102–5107.
Nisell M, Nomikos GG, Svensson TH (1994). Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 16: 36–44.
Nisenbaum ES, Berger TW (1992). Functionally distinct subpopulations of striatal neurons are differentially regulated by GABAergic and dopaminergic inputs—I. In vivo analysis. Neuroscience 48: 561–578.
Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates 5th edn Academic Press: San Diego, USA.
Perez MF, White FJ, Hu XT (2006). Dopamine D(2) receptor modulation of K(+) channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J Neurophysiol 96: 2217–2228.
Perkins KA (1995). Individual variability in responses to nicotine. Behav Genet 25: 119–132.
Picciotto MR, Corrigall WA (2002). Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci 22: 3338–3341.
Pisani A, Bonsi P, Centonze D, Calabresi P, Bernardi G (2000). Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. J Neurosci. 20: RC69.
Planert H, Szydlowski SN, Hjorth JJ, Grillner S, Silberberg G (2010). Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J Neurosci 30: 3499–3507.
Podda MV, Riccardi E, D'Ascenzo M, Azzena GB, Grassi C. (2010). Dopamine D1-like receptor activation depolarizes medium spiny neurons of the mouse nucleus accumbens by inhibiting inwardly rectifying K+ currents through a cAMP-dependent protein kinase A-independent mechanism. Neuroscience 167: 678–690.
Pomerleau OF (1995). Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet 25: 161–177.
Pontieri FE, Tanda G, Orzi F, Di Chiara G (1996). Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382: 255–257.
Rye DB, Lee HJ, Saper CB, Wainer BH (1988). Medullary and spinal efferents of the penunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol 269: 315–341.
Sellings LH, Baharnouri G, McQuade LE, Clarke PB (2008). Rewarding and aversive effects of nicotine are segregated within the nucleus accumbens. Eur J Neurosci 28: 342–352.
Smith AD, Bolam JP (1990). The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci 13: 259–265.
Sun N, Chi N, Lauzon N, Bishop S, Tan H, Laviolette SR (2011). Acquisition, extinction, and recall of opiate reward memory are signaled by dynamic neuronal activity patterns in the prefrontal cortex. Cereb Cortex 21: 2665–2680.
Sun N, Laviolette SR (2012). Inactivation of the basolateral amygdala during opiate reward learning disinhibits prelimbic cortical neurons and modulates associative memory extinction. Psychopharmacology 222: 645–661.
Taha SA, Fields HL (2005). Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci 25: 1193–1202.
Tan H, Bishop SF, Lauzon NM, Sun N, Laviolette SR (2009). Chronic nicotine exposure switches the functional role of mesolimbic dopamine transmission in the processing of nicotine's rewarding and aversive effects. Neuropharmacology 56: 741–751.
Tepper JM, Tecuapetla F, Koós T, Ibáñez-Sandoval O (2010). Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat 4: 150.
Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ (2012). Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75: 58–64.
Van Bockstaele EJ, Pickel VM. (1995). GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res 682: 215–221.
Wiltschko AB, Pettibone JR, Berke JD. (2010). Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacology 35: 1261–1270.
Yin R, French ED (2000). A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: an in vitro electrophysiological study. Brain Res Bull 51: 507–514.
Zocchi A, Girlanda E, Varnier G, Sartori I, Zanette L, Wildish GA et al (2003). Dopamine responsiveness to drugs of abuse: a shell-core investigation in the nucleus accumbens of the mouse. Synapse 50: 293–302.
Acknowledgements
This work was supported by Pfizer Canada.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, N., Laviolette, S. & Addiction Research Group. Dopamine Receptor Blockade Modulates the Rewarding and Aversive Properties of Nicotine via Dissociable Neuronal Activity Patterns in the Nucleus Accumbens. Neuropsychopharmacol 39, 2799–2815 (2014). https://doi.org/10.1038/npp.2014.130
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.130
This article is cited by
-
Modulation of Nicotine-Associated Behaviour in Rats By μ-Opioid Signals from the Medial Prefrontal Cortex to the Nucleus Accumbens Shell
Neuroscience Bulletin (2024)
-
The acute effects of nicotine on corticostriatal responses to distinct phases of reward processing
Neuropsychopharmacology (2020)
-
Nicotine regulates activity of lateral habenula neurons via presynaptic and postsynaptic mechanisms
Scientific Reports (2016)
-
Smoking Normalizes Cerebral Blood Flow and Oxygen Consumption after 12-Hour Abstention
Journal of Cerebral Blood Flow & Metabolism (2015)
-
Co-administration of ethanol and nicotine: the enduring alterations in the rewarding properties of nicotine and glutamate activity within the mesocorticolimbic system of female alcohol-preferring (P) rats
Psychopharmacology (2015)