Abstract
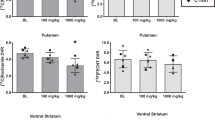
Although traditional sensitization paradigms, which result in an augmentation of cocaine-induced locomotor behavior and dopamine (DA) overflow following repeated experimenter-delivered cocaine injections, are often used as a model to study drug addiction, similar effects have been difficult to demonstrate following cocaine self-administration. We have recently shown that intermittent access (IntA) to cocaine can result in increased cocaine potency at the DA transporter (DAT); however, traditional sensitization paradigms often show enhanced effects following withdrawal/abstinence periods. Therefore, we determined a time course of IntA-induced sensitization by examining the effects of 1 or 3 days of IntA, as well as a 7-day abstinence period on DA function, cocaine potency, and reinforcement. Here we show that cocaine potency is increased following as little as 3 days of IntA and further augmented following an abstinence period. In addition, IntA plus abstinence produced greater evoked DA release in the presence of cocaine as compared with all other groups, demonstrating that following abstinence, both cocaine’s ability to increase DA release and inhibit uptake at the DAT, two separate mechanisms for increasing DA levels, are enhanced. Finally, we found that IntA-induced sensitization of the DA system resulted in an increased reinforcing efficacy of cocaine, an effect that was augmented after the 7-day abstinence period. These results suggest that sensitization of the DA system may have an important role in the early stages of drug abuse and may drive the increased drug seeking and taking that characterize the transition to uncontrolled drug use. Human data suggest that intermittency, sensitization, and periods of abstinence have an integral role in the process of addiction, highlighting the importance of utilizing pre-clinical models that integrate these phenomena, and suggesting that IntA paradigms may serve as novel models of human addiction.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmed SH, Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300.
Ahmed SH, Koob GF (1999). Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 146: 303–312.
Ahmed SH, Lenoir M, Guillem K (2013). Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol 23: 581–587.
Beveridge TJR, Wray P, Brewer A, Shapiro B, Mahoney JJ, Newton TF (2012) Analyzing Human Cocaine Use Patterns to Inform Animal Addiction Model Development. Published abstract for the College on Problems of Drug Dependence Annual Meeting. Palm Springs, CA.
Bouayad-Gervais K, Minogianis EA, Lévesque D, Samaha AN (2014). The self-administration of rapidly delivered cocaine promotes increased motivation to take the drug: contributions of prior levels of operant responding and cocaine intake. Psychopharmacology (Berl) (in press).
Calipari ES, Beveridge TJ, Jones SR, Porrino LJ (2013a). Persistent decreases in functional activity of limbic brain regions following extended access cocaine self-administration. Eur J Neurosci 38: 3749–3757.
Calipari ES, Ferris MJ, Caron MG, Roberts DC, Jones SR (2013b). Methylphenidate self-administration increases the potency and reinforcing effects of releasers through a dopamine transporter mechanism. Nat Commun 4: 2720.
Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC et al (2014a). Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict Biol 19: 145.
Calipari ES, Ferris MJ, Roberts DCS, Jones SR (2013c). Extended access cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128: 224–232.
Calipari ES, Ferris MJ, Siciliano CA, Jones SR (2014b). Intermittent cocaine self-administration produces sensitization of stimulant effects at the dopamine transporter. J Pharmacol Exp Ther 349: 192–198.
Calipari ES, Ferris MJ, Zimmer BA, Roberts DCS, Jones SR (2013d). Temporal pattern of cocaine intake determines tolerance versus sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 38: 2385–2392.
Christensen C, Silberberg A, Hursh S, Huntsberry M, Riley A (2008). Essential value of cocaine and food in rats: tests of the exponential model of demand. Psychopharmacology 198: 221–229.
Cohen Peter, Sas Arjan (1994). Cocaine use in Amsterdam in nondeviant subcultures. Addict Res 2: 71–94.
Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR (2012). Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharm 37: 1708–1716.
Ferris MJ, Calipari ES, Melchior JR, Roberts DC, España RA, Jones SR (2013b). Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur J Neurosci 38: 2628–2636.
Ferris MJ, Calipari ES, Yorgason JT, Jones SR (2013a). Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem Neurosci 4: 693–703.
Ferris MJ, Mateo Y, Roberts DC, Jones SR (2011). Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry 69: 201–207.
Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U (1989). Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens—an in vivo Microdialysis Study. Brain Res 498: 199–203.
Hursh SR, Silberberg A (2008). Economic demand and essential value. Psychol Rev 115: 186–198.
Hursh SR, Winger G (1995). Normalized demand for drugs and other reinforcers. J Exp Anal Behav 64: 373–384.
Jones SR, Lee TH, Wightman RM, Ellinwood EH (1996). Effects of intermittent and continuous cocaine administration on dopamine release and uptake regulation in the striatum: in vitro voltammetric assessment. Psychopharmacology (Berl) 126: 331–338.
Jonkman S, Pelloux Y, Everitt BJ (2012). Differential roles of the dorsolateral and midlateral striatum in punished cocaine seeking. J Neurosci 32: 4645–4650.
Kalivas PW, Duffy P (1993). Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci 13: 266–275.
Koob GF, Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science 278: 52–58.
Millan EZ, Marchant NJ, McNally GP (2011). Extinction of drug seeking. Behav Brain Res 217: 454–462.
Oleson EB, Richardson JM, Roberts DC (2011). A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl) 214: 567–577.
Oleson EB, Roberts DC (2009). Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology 34: 796–804.
Oleson EB, Roberts DC (2012). Cocaine self-administration in rats: threshold procedures. Methods Mol Biol 829: 303–319.
Ostlund SB, Leblanc KH, Kosheleff AR, Wassum KM, Maidment NT (2014). Phasic mesolimbic dopamine signaling encodes the facilitation of incentive motivation produced by repeated cocaine exposure. Neuropsychopharmacology 39: 2441–2449.
Parsons LH, Justice JB Jr (1993). Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J Neurochem 61: 1611–1619.
Pierce RC, Kalivas PW (1997). A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25: 192–216.
Post RM (1980). Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci 26: 1275–1282.
Prisciandaro JJ, Joseph JE, Myrick H, McRae-Clark AL, Henderson S, Pfeifer J et al (2014). The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues. Addiction (in press).
Robinson TE, Becker JB (1982). Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur J Pharmacol 85: 253–254.
Robinson TE, Becker JB (1986). Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res 396: 157–198.
Robinson TE, Jurson PA, Bennett JA, Bentgen KM (1988). Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res 462: 211–222.
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168: 3–20.
Siciliano CA, Calipari ES, Ferris MJ, Jones SR (2014). Biphasic mechanisms of amphetamine action at the dopamine terminal. J Neurosci 34: 5575–5582.
Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P et al (2006). Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci 26: 3206–3209.
Vezina P, Leyton M (2009). Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology 56 (Suppl 1): 160–168.
Zimmer BA, Oleson EB, Roberts DCS (2012). The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37: 1901–1910.
Acknowledgements
We would like to thank Dr Amanda Gabriele for her help with the self-administration studies in the current manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calipari, E., Siciliano, C., Zimmer, B. et al. Brief Intermittent Cocaine Self-Administration and Abstinence Sensitizes Cocaine Effects on the Dopamine Transporter and Increases Drug Seeking. Neuropsychopharmacol 40, 728–735 (2015). https://doi.org/10.1038/npp.2014.238
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.238
This article is cited by
-
Cocaine-induced plasticity, motivation, and cue responsivity do not differ in obesity-prone vs obesity-resistant rats; implications for food addiction
Psychopharmacology (2023)
-
D-amphetamine maintenance therapy reduces cocaine use in female rats
Psychopharmacology (2022)
-
Individual differences in dopamine uptake in the dorsomedial striatum prior to cocaine exposure predict motivation for cocaine in male rats
Neuropsychopharmacology (2021)
-
Dopamine transporter function fluctuates across sleep/wake state: potential impact for addiction
Neuropsychopharmacology (2021)
-
Amphetamine maintenance therapy during intermittent cocaine self-administration in rats attenuates psychomotor and dopamine sensitization and reduces addiction-like behavior
Neuropsychopharmacology (2021)