Abstract
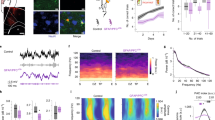
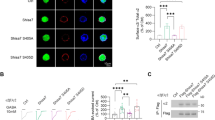
An essential aspect of goal-directed action selection is differentiating between behaviors that are more, or less, likely to be reinforced. Habits, by contrast, are stimulus-elicited behaviors insensitive to action–outcome contingencies and are considered an etiological factor in several neuropsychiatric disorders. Thus, isolating the neuroanatomy and neurobiology of goal-directed action selection on the one hand, and habit formation on the other, is critical. Using in vivo viral-mediated gene silencing, we knocked down Gabra1 in the orbitofrontal prefrontal cortex (oPFC) in mice, decreasing oPFC GABAAα1 expression, as well as expression of the synaptic marker PSD-95. Mice expressing Green Fluorescent Protein or Gabra1 knockdown in the adjacent M2 motor cortex served as comparison groups. Using instrumental response training followed by action–outcome contingency degradation, we then found that oPFC GABAAα1 deficiency impaired animals’ ability to differentiate between actions that were more or less likely to be reinforced, though sensitivity to outcome devaluation and extinction were intact. Meanwhile, M2 GABAAα1 deficiency enhanced sensitivity to action–outcome relationships. Behavioral abnormalities following oPFC GABAAα1 knockdown were rescued by testing mice in a distinct context relative to that in which they had been initially trained. Together, our findings corroborate evidence that chronic GABAAα1 deficiency remodels cortical synapses and suggest that neuroplasticity within the healthy oPFC gates the influence of reward-related contextual stimuli. These stimuli might otherwise promote maladaptive habit-based behavioral response strategies that contribute to—or exacerbate—neuropsychiatric illness.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Balleine BW, Dickinson A (1998). Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37: 407–419.
Balleine BW, Killcross AS, Dickinson A (2003). The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci 23: 666–675.
Balleine BW, O’Doherty JP (2010). Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35: 48–69.
Bouyer JJ, Park DH, Joh TH, Pickel VM (1984). Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res 302: 267–275.
Chen Y-C, Thalar D, Nixon PD, Stern CE, Passingham RE (1995). The functions of the medial premotor cortex. II. The timing and selection of learned movements. Exp Brain Res 102: 461–473.
Colwill RM, Rescorla RA (1986). Associative structures in instrumental learning. In: Bower Gordon H (ed) Psychology of Learning and Motivation Vol 20. Academic Press, Inc.: London.
Corbit LH, Balleine BW (2003). The role of prelimbic cortex in instrumental conditioning. Behav Brain Res 146: 145–157.
Corbit LH, Ostlund SB, Balleine BW (2002). Sensitivity to instrumental contingency degradation is mediated by the entorhinal cortex and its efferents via the dorsal hippocampus. J Neurosci 22: 10976–10984.
DePoy LM, Noble B, Allen AG, Gourley SL (2013). Developmentally divergent effects of Rho-kinase inhibition on cocaine- and BDNF-induced behavioral plasticity. Behav Brain Res 243: 171–175.
Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ et al (2009). Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325: 621–625.
Dickinson A (1980) Contemporary Animal Learning Theory. Cambridge University Press: Cambridge.
Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF (2006). Representation of spatial goals in rat orbitofrontal cortex. Neuron 51: 495–507.
Fuchs RA, Evans KA, Parker MP, See RE (2004). Differential involvement of the orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci 24: 6600–6610.
Gourley SL, Howell JL, Rios M, DiLeone RJ, Taylor JR (2009). Prelimbic cortex bdnf knock-down reduces instrumental responding in extinction. Learn Mem 16: 756–760.
Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR (2010). Dissociable regulation of goal-directed action within mouse prefrontal cortex. Eur J Neurosci 32: 1726–1734.
Gourley SL, Olevska A, Gordon J, Taylor JR (2013b). Cytoskeletal determinants of stimulus-response habits. J Neurosci 33: 11811–11816.
Gourley SL, Olevska A, Zimmermann KS, Ressler KJ, DiLeone RJ, Taylor JR (2013a). The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur J Neurosci 38: 2382–2388.
Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, Dileone RJ et al (2012). Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci USA 109: 20714–20719.
Grabenhorst F, Rolls ET (2011). Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci 15: 56–67.
Gremel CM, Costa RM (2013). Premotor cortex is critical for goal-directed action. Front Comp Neurosci 7: 110.
Hammond LJ (1980). The effect of contingency upon the appetitive conditioning of free-operant behavior. J Exp Anal Behav 34: 297–304.
Heinen K, Baker RE, Spijker S, Rosahl T, van Pelt J, Brussaard AB (2003). Impaired dendritic spine maturation in GABAA receptor alpha1 subunit knock out mice. Neuroscience 122: 699–705.
Heldt SA, Ressler KJ (2010). Amygdala-specific reduction of alpha1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J Neurosci 30: 7139–7151.
Hines RM, Davies PA, Moss SJ, Maguire J (2012). Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol 22: 552–558.
Hinton EA, Wheeler MG, Gourley SL (2014). Early-life cocaine interferes with BDNF-mediated behavioral plasticity. Learn Mem 21: 253–257.
Killcross S, Coutoureau E (2003). Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex 13: 400–408.
Kita H, Kitai ST (1990). Amygdaloid projection to the frontal cortex and the striatum in the rat. J Comp Neurol 298: 40–49.
Kralic JE, Korpi ER, O’Buckley TK, Homanics GE, Morrow AL (2002). Molecular and pharmacological characterization of GABA(A) receptor alpha1 subunit knockout mice. J Pharmacol Exp Ther 302: 1037–1045.
Lasseter HC, Ramirez DR, Xie X, Fuchs RA (2009). Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci 30: 1370–1381.
Lasseter HC, Xie X, Arguello AA, Wells AM, Hodges MA, Fuchs RA (2014). Contribution of a mesocorticolimbic subcircuit to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 39: 660–669.
Lex B, Hauber W (2010). Disconnection of the entorhinal cortex and dorsomedial striatum impairs the sensitivity to instrumental contingency degradation. Neuropsychopharmacology 35: 1788–1796.
Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM (2010). Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron 67: 821–833.
Lu H, Lim B, Poo MM (2009). Cocaine exposure in utero alters synaptic plasticity in the medial prefrontal cortex of postnatal rats. J Neurosci 29: 12664–12674.
Ongur D, Price JL (2000). The organization of networks within the orbital and medial prefrontal cortex in rats, monkeys, and humans. Cereb Cortex 10: 206–219.
Ostlund SB, Balline BW (2007). Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci 27: 4819–4825.
Overmier JB, Linwick D (2001). Conditional choice-unique outcomes establish expectancies that mediate choice behavior. Integr Physiol Behav Sci 36: 173–181.
Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR (2007). Sex chromosome complement regulates habit formation. Nat Neurosci 10: 1398–1400.
Rosen G, Williams AG, Capra JA, Connolly MT, Cruz B, Lu L et al (2000). The mouse brain library @ www.Mbl.Org. International M ouse Genome Conference; 14, 166.
Schwabe L, Dickinson A, Wolf OT (2011). Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol 19: 53–63.
Schwabe L, Wolf OT (2013). Stress and multiple memory systems: from ‘thinking’ to ‘doing’. Trends Cogn Sci 17: 60–68.
Skilbeck KJ, Johnston GAR, Hinton T (2010). Stress and GABAA receptors. J Neurochem 112: 1115–1130.
Sonner JM, Cascio M, Xing Y, Fanselow MS, Kralic JE, Morrow AL et al (2005). Alpha1 subunit-contain GABA type A receptors in forebrain contribute to the effects of inhaled anesthetics on conditioned fear. Mol Pharmacol 68: 61–68.
Swanson AM, Shapiro LP, Whyte AJ, Gourley SL (2013). Glucocorticoid receptor regulation of action selection and prefrontal cortical dendritic spines. Commun Integr Biol 6: e26068.
Tanji J, Shima K, Mushiake H (1996). Multiple cortical motor areas and temporal sequencing of movements. Brain Res Cogn Brain Res 1-2: 117–122.
Trapold MA (1970). Are expectancies based upon different positive reinforcing events discriminably different? Learn Motiv 1: 129–140.
Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE (2001). GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci 21: 3009–3016.
Yin HH (2009). The role of the murine motor cortex in action duration and order. Front Integr Neurosci 3: 23.
Zhou C, Huang Z, Ding L, Deel ME, Arain FM, Murray CR et al (2013). Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome. J Biol Chem 288: 21458–21472.
Acknowledgements
We thank Mr Alonzo Whyte for assistance, Ms Kelsey Zimmermann and Dr Donald Rainnie for feedback, and Dr Kerry Ressler for advice and for generously providing the Gabra1-tm1Geh mice used here.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swanson, A., Allen, A., Shapiro, L. et al. GABAAα1-Mediated Plasticity in the Orbitofrontal Cortex Regulates Context-Dependent Action Selection. Neuropsychopharmacol 40, 1027–1036 (2015). https://doi.org/10.1038/npp.2014.292
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.292
This article is cited by
-
Anatomical specialties for value information
Nature Neuroscience (2019)
-
The Glutamatergic Postrhinal Cortex–Ventrolateral Orbitofrontal Cortex Pathway Regulates Spatial Memory Retrieval
Neuroscience Bulletin (2019)
-
Bidirectional coordination of actions and habits by TrkB in mice
Scientific Reports (2018)
-
Memory Retention Involves the Ventrolateral Orbitofrontal Cortex: Comparison with the Basolateral Amygdala
Neuropsychopharmacology (2018)
-
Successful pharmacotherapy for the treatment of severe feeding aversion with mechanistic insights from cross-species neuronal remodeling
Translational Psychiatry (2017)