Abstract
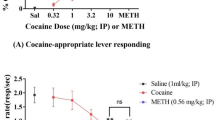
A new pharmacokinetic approach treating cocaine addiction involves rapidly metabolizing cocaine before it reaches brain reward centers using mutated human butyrylcholinesterase (BChE) or cocaine hydrolase (CocH). Recent work has shown that helper-dependent adenoviral (hdAD) vector-mediated plasma CocH reduced the locomotor-activating effects of cocaine and prevented reinstatement of cocaine-seeking behavior up to 6 months in rats. The present study investigated whether hdAD-CocH could decrease ongoing intravenous cocaine (0.4 mg/kg) self-administration. The hdAD-CocH vector was injected into self-administering rats, and after accumulation of plasma CocH, there was a dramatic reduction in cocaine infusions earned under a fixed ratio 1 schedule of reinforcement that lasted for the length of the study (>2 months). Pretreatment with the selective BChE and CocH inhibitor iso-OMPA (1.5 mg/kg) restored cocaine intake; therefore, the decline in self-administration was likely due to rapid CocH-mediated cocaine metabolism. Direct measurements of cocaine levels in plasma and brain samples taken after the conclusion of behavioral studies provided strong support for this conclusion. Further, rats injected with hdAD-CocH did not experience a deficit in operant responding for drug reinforcement and self-administered methamphetamine (0.05 mg/kg) at control levels. Overall, these outcomes suggest that viral gene transfer can yield plasma CocH levels that effectively diminish long-term cocaine intake and may have potential treatment implications for cocaine-dependent individuals seeking to become and remain abstinent.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anker JJ, Baron TR, Zlebnik NE, Carroll ME (2012a). Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend 124: 149–153.
Anker JJ, Brimijoin S, Gao Y, Geng L, Zlebnik NE, Parks RJ et al (2012b). Cocaine hydrolase encoded in viral vector blocks the reinstatement of cocaine seeking in rats for 6 months. Biol Psychiatry 71: 700–705.
Anker JJ, Carroll ME (2011). Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Topics Behav Neurosci 8: 73–96.
Austin L, Berry WK (1953). Two selective inhibitors of cholinesterase. Biochem J 54: 695–700.
Berridge CW (2006). Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology 31: 2332–2340.
Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R et al (2008). A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology 33: 2715–2725.
Brimijoin S, Orson F, Kosten TR, Kinsey B, Shen XY, White SJ et al (2013). Anti-cocaine antibody and butyrylcholinesterase-derived cocaine hydrolase exert cooperative effects on cocaine pharmacokinetics and cocaine-induced locomotor activity in mice. Chem Biol Interact 203: 212–216.
Brimijoin S, Shen ML, Sun H (2002). Radiometric solvent-partitioning assay for screening cocaine hydrolases and measuring cocaine levels in milligram tissue samples. Anal Biochem 309: 200–205.
Carroll ME, Anker JJ (2010). Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav 58: 44–56.
Carroll ME, Boe IN (1982). Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav 17: 563–567.
Carroll ME, Gao Y, Brimijoin S, Anker JJ (2011). Effects of cocaine hydrolase on cocaine self-administration under a PR schedule and during extended access (escalation) in rats. Psychopharmacology (Berl) 213: 817–829.
Carroll ME, Zlebnik NE, Anker JJ, Kosten TR, Orson FM, Shen X et al (2012). Combined cocaine hydrolase gene transfer and anti-cocaine vaccine synergistically block cocaine-induced locomotion. PLoS One 7: e43536.
Crumb WJ Jr., Clarkson CW (1992). Characterization of the sodium channel blocking properties of the major metabolites of cocaine in single cardiac myocytes. J Pharmacol Exp Ther 261: 910–917.
Gao Y, Atanasova E, Sui N, Pancook JD, Watkins JD, Brimijoin S (2005). Gene transfer of cocaine hydrolase suppresses cardiovascular responses to cocaine in rats. Mol Pharmacol 67: 204–211.
Gao Y, Brimijoin S (2004). An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther 310 1046–1052.
Gao Y, Brimijoin S (2006). Viral transduction of cocaine hydrolase in brain reward centers. Cell Mol Neurobiol 26: 357–363.
Gao Y, Orson FM, Kinsey B, Kosten T, Brimijoin S (2010). The concept of pharmacologic interception as a treatment for drug abuse. Chem Biol Interact 187: 421–424.
Geng L, Gao Y, Chen X, Hou S, Zhan CG, Radic Z et al (2013). Gene transfer of mutant mouse cholinesterase provides high lifetime expression and reduced cocaine responses with no evident toxicity. PLoS One 8: e67446.
Grubic Z, Sket D, Brzin M (1988). Iso-OMPA-induced potentiation of soman toxicity in rat correlates with the inhibition of plasma carboxylesterases. Arch Toxicol 62: 398–399.
Inaba T (1989). Cocaine: pharmacokinetics and biotransformation in man. Can J Physiol Pharmacol 67: 1154–1157.
Johanson CE, Fischman MW (1989). The pharmacology of cocaine related to its abuse. Pharmacol Rev 41: 3–52.
Kalow W (2004). Human pharmacogenomics: the development of a science. Hum Genomics 1: 375–380.
Mesulam M (2003). Butyrylcholinesterase in the normal and Alzheimer brain. In: Giacobini E (ed) Butyrylcholinesterase, Its Function and Inhibitors. Martin Dunitz: New York. pp 29–37.
Murthy V, Gao Y, Geng L, Lebrasseur N, White T, Brimijoin S (2013). Preclinical studies on neurobehavioral and neuromuscular effects of cocaine hydrolase gene therapy in mice. J Mol Neurosci, (2013). PMID: 24085526 (doi:10.1007/s12031-013-0130-5).
National Research Council (2011) Guide for the Care and Use of Animals 8th edn The National Academies Press: Washington, DC.
Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH et al (2005). Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci USA 102: 16656–16661.
Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL (1996). A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA 93: 13565–13570.
Seiden LS, Sabol KE, Ricaurte GA (1993). Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol 33: 639–677.
Soliday FK, Conley YP, Henker R (2010). Pseudocholinesterase deficiency: a comprehensive review of genetic, acquired, and drug influences. Aana J 78: 313–320.
Sun H, Pang YP, Lockridge O, Brimijoin S (2002). Re-engineering butyrylcholinesterase as a cocaine hydrolase. Mol Pharmacol 62: 220–224.
Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY et al (1994). The demethylenation of methylenedioxymethamphetamine (‘ecstasy’) by debrisoquine hydroxylase (CYP2D6). Biochem Pharmacol 47: 1151–1156.
Weber A, Butterweck H, Mais-Paul U, Teschner W, Lei L, Muchitsch EM et al (2011). Biochemical, molecular and preclinical characterization of a double-virus-reduced human butyrylcholinesterase preparation designed for clinical use. Vox Sang 100: 285–297.
Xue L, Hou S, Yang W, Fang L, Zheng F, Zhan CG (2013). Catalytic activities of a cocaine hydrolase engineered from human butyrylcholinesterase against (+)- and (−)-cocaine. Chem Biol Interact 203: 57–62.
Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D et al (2008). Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc 130: 12148–12155.
Zlebnik NE, Anker JJ, Gliddon LA, Carroll ME (2010). Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology 209: 113–125.
Acknowledgements
We thank Cole Batty, James Brown, Luke Bushman, Clare Chamberlain, Seth Johnson, Sarah Korthauer, Torie Lepak, Nathan Omdalen, Heather Veglahn, and Ashley Xiong for technical assistance and Krista Walkowiak, DVM, for veterinary care. Funding for this study was provided by the National Institute on Drug Abuse (NIDA) grant DP1 DA031340 (SB); NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zlebnik, N., Brimijoin, S., Gao, Y. et al. Long-Term Reduction of Cocaine Self-Administration in Rats Treated with Adenoviral Vector-Delivered Cocaine Hydrolase: Evidence for Enzymatic Activity. Neuropsychopharmacol 39, 1538–1546 (2014). https://doi.org/10.1038/npp.2014.3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.3
Keywords
This article is cited by
-
In vitro and in vivo stability of a highly efficient long-acting cocaine hydrolase
Scientific Reports (2024)
-
Environmental, genetic and epigenetic contributions to cocaine addiction
Neuropsychopharmacology (2018)
-
Plant-expressed cocaine hydrolase variants of butyrylcholinesterase exhibit altered allosteric effects of cholinesterase activity and increased inhibitor sensitivity
Scientific Reports (2017)
-
Reaction pathway for cocaine hydrolase-catalyzed hydrolysis of (+)-cocaine
Theoretical Chemistry Accounts (2016)
-
Reward and Toxicity of Cocaine Metabolites Generated by Cocaine Hydrolase
Cellular and Molecular Neurobiology (2015)