Abstract
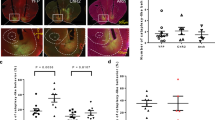
The nucleus accumbens (NAc) contains a hedonic hotspot in the rostral half of medial shell, where opioid agonist microinjections are known to enhance positive hedonic orofacial reactions to the taste of sucrose (‘liking’ reactions). Within NAc shell, orexin/hypocretin also has been reported to stimulate food intake and is implicated in reward, whereas blockade of muscarinic acetylcholine receptors by scopolamine suppresses intake and may have anti-reward effects. Here, we show that NAc microinjection of orexin-A in medial shell amplifies the hedonic impact of sucrose taste, but only within the same anatomically rostral site, identical to the opioid hotspot. By comparison, at all sites throughout medial shell, orexin microinjections stimulated ‘wanting’ to eat, as reflected by increases in intake of palatable sweet chocolates. At NAc shell sites outside the hotspot, orexin selectively enhanced ‘wanting’ to eat without enhancing sweetness ‘liking’ reactions. In contrast, microinjections of the antagonist scopolamine at all sites in NAc shell suppressed sucrose ‘liking’ reactions as well as suppressing intake of palatable food. Conversely, scopolamine increased aversive ‘disgust’ reactions elicited by bitter quinine at all NAc shell sites. Finally, scopolamine microinjections localized to the caudal half of medial shell additionally generated a fear-related anti-predator reaction of defensive treading and burying directed toward the corners of the transparent chamber. Together, these results confirm a rostral hotspot in NAc medial shell as a unique site for orexin induction of hedonic ‘liking’ enhancement, similar to opioid enhancement. They also reveal distinct roles for orexin and acetylcholine signals in NAc shell for hedonic reactions and motivated behaviors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Avena NM, Rada P, Hoebel BG (2008). Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience 156: 865–871.
Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003). Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464: 220–237.
Barson JR, Ho HT, Leibowitz SF (2015). Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol 20: 469–481.
Berridge KC (2000). Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev 24: 173–198.
Berridge KC, Kringelbach ML (2015). Pleasure systems in the brain. Neuron 86: 646–664.
Berthoud HR, Munzberg H (2011). The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav 104: 29–39.
Castro DC, Berridge KC (2014). Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness ‘liking’ and ‘wanting’. J Neurosci 34: 4239–4250.
Ch'ng SS, Lawrence AJ (2015). Distribution of the orexin-1 receptor (OX1R) in the mouse forebrain and rostral brainstem: a characterisation of OX1R-eGFP mice. J Chem Neuroanat 66-67: 1–9.
Clarke SN, Ossenkopp KP (1998). Taste reactivity responses in rats: influence of sex and the estrous cycle. Am J Physiol 274: R718–R724.
Coss RG, Owings DH (1978). Snake-directed behavior by snake naive and experienced California ground squirrels in a simulated burrow. Z Tierpsychol 48: 421–435.
Covelo IR, Patel ZI, Luviano JA, Stratford TR, Wirtshafter D (2014). Manipulation of GABA in the ventral pallidum, but not the nucleus accumbens, induces intense, preferential, fat consumption in rats. Behav Brain Res 270: 316–325.
DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC (2012). Enkephalin surges in dorsal neostriatum as a signal to eat. Curr Biol 22: 1918–1924.
Espana RA, Valentino RJ, Berridge CW (2003). Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience 121: 201–217.
Faure A, Richard JM, Berridge KC (2010). Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS One 5: e11223.
Flynn FW, Schulkin J, Havens M (1993). Sex differences in salt preference and taste reactivity in rats. Brain Res Bull 32: 91–95.
Grill HJ, Norgren R (1978). The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143: 263–279.
Harris GC, Wimmer M, Aston-Jones G (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556–559.
Ho CY, Berridge KC (2013). An orexin hotspot in ventral pallidum amplifies hedonic 'liking' for sweetness. Neuropsychopharmacology 38: 1655–1664.
Ho CY, Berridge KC (2014). Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. Eur J Neurosci 40: 3556–3572.
Kelley AE, Baldo BA, Pratt WE (2005). A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493: 72–85.
Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE (2003). Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci 23: 7–11.
Mahler SV, Berridge KC (2009). Which cue to ‘want?’ Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29: 6500–6513.
Mahler SV, Smith KS, Berridge KC (2007). Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward. Neuropsychopharmacology 32: 2267–2278.
Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995). Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15: 6779–6788.
Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M et al (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25.
Mark GP, Rada P, Pothos E, Hoebel BG (1992). Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum, and hippocampus of freely behaving rats. J Neurochem 58: 2269–2274.
Mark GP, Weinberg JB, Rada PV, Hoebel BG (1995). Extracellular acetylcholine is increased in the nucleus accumbens following the presentation of an aversively conditioned taste stimulus. Brain Res 688: 184–188.
Martin G, Fabre V, Siggins GR, de Lecea L (2002). Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept 104: 111–117.
Mena JD, Sadeghian K, Baldo BA (2011). Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci 31: 3249–3260.
Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE (2008). The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct 213: 17–27.
Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H (2004). Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43: 133–143.
Patyal R, Woo EY, Borgland SL (2012). Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front Behav Neurosci 6: 82.
Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates. Academic: New York.
Pecina S, Berridge KC (2005). Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 25: 11777–11786.
Pecina S, Berridge KC (2013). Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered 'wanting' for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci 37: 1529–1540.
Perry ML, Baldo BA, Andrzejewski ME, Kelley AE (2009). Muscarinic receptor antagonism causes a functional alteration in nucleus accumbens mu-opiate-mediated feeding behavior. Behav Brain Res 197: 225–229.
Perry ML, Pratt WE, Baldo BA (2014). Overlapping striatal sites mediate scopolamine-induced feeding suppression and mu-opioid-mediated hyperphagia in the rat. Psychopharmacology (Berl) 231: 919–928.
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG et al (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015.
Pratt WE, Kelley AE (2004). Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behav Neurosci 118: 730–739.
Pratt WE, Kelley AE (2005). Striatal muscarinic receptor antagonism reduces 24-h food intake in association with decreased preproenkephalin gene expression. Eur J Neurosci 22: 3229–3240.
Pratt WE, Spencer RC, Kelley AE (2007). Muscarinic receptor antagonism of the nucleus accumbens core causes avoidance to flavor and spatial cues. Behav Neurosci 121: 1215–1223.
Ragnauth A, Moroz M, Bodnar RJ (2000). Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res 876: 76–87.
Reynolds SM, Berridge KC (2001). Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci 21: 3261–3270.
Reynolds SM, Berridge KC (2002). Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste ‘liking’/’disliking’ reactions, place preference/avoidance, and fear. J Neurosci 22: 7308–7320.
Reynolds SM, Berridge KC (2008). Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci 11: 423–425.
Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC (2013). Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley. Neurosci Biobehav Rev 37: 1919–1931.
Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC et al (2011). Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA 108: 13305–13310.
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H et al (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585.
Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ (2010). Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry 67: 753–760.
Shinohara Y, Inui T, Yamamoto T, Shimura T (2009). Cannabinoid in the nucleus accumbens enhances the intake of palatable solution. Neuroreport 20: 1382–1385.
Smith KS, Berridge KC (2005). The ventral pallidum and hedonic reward: neurochemical maps of sucrose ‘liking’ and food intake. J Neurosci 25: 8637–8649.
Smith KS, Berridge KC, Aldridge JW (2011). Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA 108: E255–E264.
Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L et al (2007). Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol 151: 1109–1116.
Steiner JE (1973). The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept 4: 254–278.
Steiner JE, Glaser D, Hawilo ME, Berridge KC (2001). Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev 25: 53–74.
Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L et al (2015). Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun 6: 8543.
Sutcliffe JG, de Lecea L (2002). The hypocretins: setting the arousal threshold. Nat Rev Neurosci 3: 339–349.
Sweet DC, Levine AS, Kotz CM (2004). Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides 25: 307–314.
Thompson RH, Swanson LW (2010). Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci USA 107: 15235–15239.
Thorpe AJ, Kotz CM (2005). Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res 1050: 156–162.
Treit D, Pinel JP, Fibiger HC (1981). Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav 15: 619–626.
Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM (1998). Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438: 71–75.
van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB (2002). Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol 541: 169–185.
Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P et al (2010). Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330: 1677–1681.
Zahm DS, Parsley KP, Schwartz ZM, Cheng AY (2013). On lateral septum-like characteristics of outputs from the accumbal hedonic ‘hotspot’ of Pecina and Berridge with commentary on the transitional nature of basal forebrain ‘boundaries’. J Comp Neurol 521: 50–68.
Zhang M, Kelley AE (2000). Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience 99: 267–277.
Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y et al (2003). Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci 92: 259–266.
Acknowledgements
We thank Cristina Muñoz, Cody Schember, and Josh Goldman for their help with immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, D., Terry, R. & Berridge, K. Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose ‘Liking’ and Intake but Scopolamine in Caudal Shell Shifts ‘Liking’ Toward ‘Disgust’ and ‘Fear’. Neuropsychopharmacol 41, 2101–2111 (2016). https://doi.org/10.1038/npp.2016.10
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.10
This article is cited by
-
Sleep-mediated regulation of reward circuits: implications in substance use disorders
Neuropsychopharmacology (2023)
-
Orexin-1 receptor signaling in ventral tegmental area mediates cue-driven demand for cocaine
Neuropsychopharmacology (2022)
-
Sex differences in activation of extra-hypothalamic forebrain areas during hedonic eating
Brain Structure and Function (2022)
-
Anxiety and cognitive-related effects of Δ 9-tetrahydrocannabinol (THC) are differentially mediated through distinct GSK-3 vs. Akt-mTOR pathways in the nucleus accumbens of male rats
Psychopharmacology (2022)
-
An endogenous opioid circuit determines state-dependent reward consumption
Nature (2021)