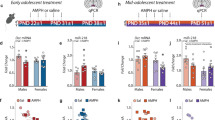
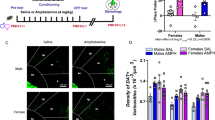
Abstract
The development of the dopamine input to the medial prefrontal cortex occurs during adolescence and is a process that is vulnerable to disruption by stimulant drugs such as amphetamine. We have previously linked the amphetamine-induced disruption of dopamine connectivity and prefrontal cortex maturation during adolescence to the downregulation of the Netrin-1 receptor, DCC, in dopamine neurons. However, how DCC expression in dopamine neurons is itself regulated is completely unknown. MicroRNA (miRNA) regulation of mRNA translation and stability is a prominent mechanism linking environmental events to changes in protein expression. Here, using male mice, we show that miR-218 is expressed in dopamine neurons and is a repressor of DCC. Whereas Dcc mRNA levels increase from early adolescence to adulthood, miR-218 exhibits the exact opposite switch, most likely maintaining postnatal Dcc expression. This dynamic regulation appears to be selective to Dcc since the expression of Robo 1, the other guidance cue receptor target of miR-218, does not vary with age. Amphetamine in adolescence, but not in adulthood, increases miR-218 in the VTA and this event is required for drug-induced downregulation of Dcc mRNA and protein expression. This effect seems to be specific to Dcc because amphetamine does not alter Robo1. Furthermore, the upregulation of miR-218 by amphetamine requires dopamine D2 receptor activation. These findings identify miR-218 as regulator of DCC in the VTA both in normal development and after drug exposure in adolescence.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aranda PS, LaJoie DM, Jorcyk CL (2012). Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis 33: 366–369.
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G et al (2014). Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci 17: 131–143.
Chakraborty C, Das S (2016). Profiling cell-free and circulating miRNA: a clinical diagnostic tool for different cancers. Tumour Biol 37: 5705–5714.
Codocedo JF, Inestrosa NC (2016). Environmental control of microRNAs in the nervous system: Implications in plasticity and behavior. Neurosci Biobehav Rev 60: 121–138.
Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F, Ron D (2015). MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Mol Psychiatry 20: 1261.
Dudanova I, Klein R (2013). Integration of guidance cues: parallel signaling and crosstalk. Trends Neurosci 36: 295–304.
Eipper-Mains JE, Kiraly DD, Palakodeti D, Mains RE, Eipper BA, Graveley BR (2011). microRNA-Seq reveals cocaine-regulated expression of striatal microRNAs. RNA 17: 1529–1543.
Fan X, Hess EJ (2007). D2-like dopamine receptors mediate the response to amphetamine in a mouse model of ADHD. Neurobiol Dis 26: 201–211.
Flores C (2011). Role of netrin-1 in the organization and function of the mesocorticolimbic dopamine system. J Psychiatry Neurosci 36: 296–310.
Goldman JS, Ashour MA, Magdesian MH, Tritsch NX, Harris SN, Christofi N et al (2013). Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. J Neurosci 33: 17278–17289.
Grant BF, Dawson DA (1998). Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 10: 163–173.
Han F, Konkalmatt P, Chen J, Gildea J, Felder RA, Jose PA et al (2015). MiR-217 mediates the protective effects of the dopamine D2 receptor on fibrosis in human renal proximal tubule cells. Hypertension 65: 1118–1125.
He M, Liu Y, Wang X, Zhang MQ, Hannon GJ, Huang ZJ (2012). Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron 73: 35–48.
Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q et al (2010). Striatal microRNA controls cocaine intake through CREB signalling. Nature 466: 197–202.
Hollins SL, Cairns MJ (2016). MicroRNA: small RNA mediators of the brains genomic response to environmental stress. Prog Neurobiol 143: 61–81.
Im HI, Hollander JA, Bali P, Kenny PJ (2010). MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13: 1120–1127.
Issler O, Chen A (2015). Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci 16: 201–212.
Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R et al (2014). MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 83: 344–360.
Jin XF, Wu N, Wang L, Li J (2013). Circulating microRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell Mol Neurobiol 33: 601–613.
Joilin G, Guevremont D, Ryan B, Claudianos C, Cristino AS, Abraham WC et al (2014). Rapid regulation of microRNA following induction of long-term potentiation in vivo. Front Mol Neurosci 7: 98.
Kalivas PW, Duffy P (1991). Comparison of somatodendritic and axonal mesolimbic dopamine release using in vivo dialysis. J Neurochem 56: 961–967.
Krutzfeldt J, Kuwajima S, Braich R, Rajeev KG, Pena J, Tuschl T et al (2007). Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res 35: 2885–2892.
Kucherenko MM, Barth J, Fiala A, Shcherbata HR (2012). Steroid-induced microRNA let-7 acts as a spatio-temporal code for neuronal cell fate in the developing Drosophila brain. EMBO J 31: 4511–4523.
Laviola G, Macri S, Morley-Fletcher S, Adriani W (2003). Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev 27: 19–31.
Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J (2008). Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57: 760–773.
Manitt C, Eng C, Pokinko M, Ryan RT, Torres-Berrio A, Lopez JP et al (2013). DCC orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients. Transl Psychiatry 3: e338.
Manitt C, Labelle-Dumais C, Eng C, Grant A, Mimee A, Stroh T et al (2010). Peri-pubertal emergence of UNC-5 homologue expression by dopamine neurons in rodents. PLoS ONE 5: e11463.
Manitt C, Mimee A, Eng C, Pokinko M, Stroh T, Cooper HM et al (2011). The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J Neurosci 31: 8381–8394.
Mansour A, Meador-Woodruff JH, Zhou Q, Civelli O, Akil H, Watson SJ (1992). A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience 46: 959–971.
Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chedotal A (2002). Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol 442: 130–155.
McCabe SE, West BT, Morales M, Cranford JA, Boyd CJ (2007). Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction 102: 1920–1930.
Money KM, Stanwood GD (2013). Developmental origins of brain disorders: roles for dopamine. Front Cell Neurosci 7: 260.
Mukherji S, Ebert MS, Zheng GX, Tsang JS, Sharp PA, van Oudenaarden A (2011). MicroRNAs can generate thresholds in target gene expression. Nat Genet 43: 854–859.
Nolan K, Mitchem MR, Jimenez-Mateos EM, Henshall DC, Concannon CG, Prehn JH (2014). Increased expression of microRNA-29a in ALS mice: functional analysis of its inhibition. J Mol Neurosci 53: 231–241.
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press: NY.
Prins SA, Przybycien-Szymanska MM, Rao YS, Pak TR (2014). Long-term effects of peripubertal binge EtOH exposure on hippocampal microRNA expression in the rat. PLoS ONE 9: e83166.
Rao P, Benito E, Fischer A (2013a). MicroRNAs as biomarkers for CNS disease. Front Mol Neurosci 6: 39.
Rao YS, Mott NN, Wang Y, Chung WC, Pak TR (2013b). MicroRNAs in the aging female brain: a putative mechanism for age-specific estrogen effects. Endocrinology 154: 2795–2806.
Rao YS, Pak TR (2016). microRNAs and the adolescent brain: filling the knowledge gap. Neurosci Biobehav Rev 70: 313–322.
Rao YS, Shults CL, Pinceti E, Pak TR (2015). Prolonged ovarian hormone deprivation alters the effects of 17beta-estradiol on microRNA expression in the aged female rat hypothalamus. Oncotarget 6: 36965–36983.
Reynolds LM, Makowski CS, Yogendran SV, Kiessling S, Cermakian N, Flores C (2015). Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner. Neuropsychopharmacology 40: 1101–1112.
Reynolds LM, Pokinko M, Torres-Berrio A, Cuesta S, Lambert LC, Del Cid Pellitero E et al (2017). DCC receptors drive prefrontal cortex maturation by determining dopamine axon targeting in adolescence. Biol Psychiatry. (in press); doi: 10.1016/j.biopsych.2017.06.009.
Sambandan S, Akbalik G, Kochen L, Rinne J, Kahlstatt J, Glock C et al (2017). Activity-dependent spatially localized miRNA maturation in neuronal dendrites. Science 355: 634–637.
Schneider M (2013). Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res 354: 99–106.
Sim SE, Bakes J, Kaang BK (2014). Neuronal activity-dependent regulation of microRNAs. Mol Cells 37: 511–517.
Small EM, Sutherland LB, Rajagopalan KN, Wang S, Olson EN (2010). MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ Res 107: 1336–1344.
Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463.
Sun AX, Crabtree GR, Yoo AS (2013). MicroRNAs: regulators of neuronal fate. Curr Opin Cell Biol 25: 215–221.
Swendsen J, Burstein M, Case B, Conway KP, Dierker L, He J et al (2012). Use and abuse of alcohol and illicit drugs in US adolescents: results of the National Comorbidity Survey-Adolescent Supplement. Arch Gen Psychiatry 69: 390–398.
Thiebes KP, Nam H, Cambronne XA, Shen R, Glasgow SM, Cho HH et al (2015). miR-218 is essential to establish motor neuron fate as a downstream effector of Isl1-Lhx3. Nat Commun 6: 7718.
Tirelli E, Laviola G, Adriani W (2003). Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev 27: 163–178.
Torres-Berrío A, Lopez JP, Bagot RC, Nouel D, Dal BoG, Cuesta S et al (2017). DCC confers susceptibility to depression-like behaviors in humans and mice and is regulated by miR-218. Biol Psychiatry 81: 306–315.
Vezina P (2004). Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychostimulant drugs. Neurosci Biobehav Rev 27: 827–839.
Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ et al (2010). The microRNA spectrum in 12 body fluids. Clin Chem 56: 1733–1741.
Yan B, Hu Z, Yao W, Le Q, Xu B, Liu X et al (2017). MiR-218 targets MeCP2 and inhibits heroin seeking behavior. Sci Rep 7: 40413.
Yetnikoff L, Almey A, Arvanitogiannis A, Flores C (2011). Abolition of the behavioral phenotype of adult netrin-1 receptor deficient mice by exposure to amphetamine during the juvenile period. Psychopharmacology (Berl) 217: 505–514.
Yetnikoff L, Eng C, Benning S, Flores C (2010). Netrin-1 receptor in the ventral tegmental area is required for sensitization to amphetamine. Eur J Neurosci 31: 1292–1302.
Acknowledgements
Author contributions
SC and CF conceived the experiments. SC, AA, and CF designed the experiments. SC and JMR-L performed the developmental neuroanatomical and molecular characterization, drug, and antagomir experiments. SS performed all the stereotaxic surgeries. DN contributed to the in situ hybridization experiments. AT-B and LMR provided technical training necessary to perform part of the experiments. SC, JMR-L, AA and CF analyzed the results. SC, AA and CF wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cuesta, S., Restrepo-Lozano, J., Silvestrin, S. et al. Non-Contingent Exposure to Amphetamine in Adolescence Recruits miR-218 to Regulate Dcc Expression in the VTA. Neuropsychopharmacol. 43, 900–911 (2018). https://doi.org/10.1038/npp.2017.284
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.284
This article is cited by
-
Divergent outcomes of delta 9 – tetrahydrocannabinol (THC) in adolescence on mesocortical dopamine and cognitive development in male and female mice
Psychopharmacology (2025)
-
Rewiring the future: drugs abused in adolescence may predispose to mental illness in adult life by altering dopamine axon growth
Journal of Neural Transmission (2024)
-
Amphetamine disrupts dopamine axon growth in adolescence by a sex-specific mechanism in mice
Nature Communications (2023)
-
MiR-218: a molecular switch and potential biomarker of susceptibility to stress
Molecular Psychiatry (2020)
-
Guidance cues: linking drug use in adolescence with psychiatric disorders
Neuropsychopharmacology (2019)