Abstract
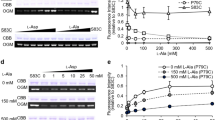
The effects of alanine substitutions in each helical segment of the structure, and Gly to D-Ala mutations at sites where glycines have positive phi angles in the Trp-cage miniprotein are reported. The effects of the stabilizing mutation were additive, yielding a 20-residue construct (Tm = 83^o^C). Gly to L-Ala substitutions were uniformly destabilizing ([DELTA][DELTA]G~F~ > 11 kJ/mol): the preference for a D-Ala can be as large as 16 kJ/mol. Glycine to D-Ala mutations are validated as a strategy for the design of hyperstable miniprotein scaffolds suitable for stereospecific pharmacophore display.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, D., Barua, B. & Andersen, N. A Hyperstable Miniprotein: Additive Effects of D- and L-Ala Substitutions. Nat Prec (2008). https://doi.org/10.1038/npre.2008.2074.1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/npre.2008.2074.1