Abstract
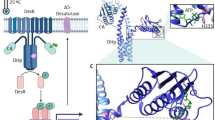
The nuclear retinoic acid (RA) receptor alpha (RAR&x03B1;) is a transcriptional transregulator that controls the expression of specific gene subsets through binding at response elements and dynamic interactions with coregulators, which are coordinated by the ligand. Here, we highlighted a novel paradigm in which the transcription of RAR&x03B1;-target genes is controlled by phosphorylation cascades initiated by the rapid RA activation of the p38MAPK/MSK1 pathway. We demonstrate that MSK1 phosphorylates RAR&x03B1; at S369 located in the Ligand Binding Domain, allowing the binding of TFIIH and thereby phosphorylation of the N-terminal domain at S77 by cdk7/cyclin H. MSK1 also phosphorylates Histone H3 at S10. Finally, the phosphorylation cascade initiated by MSK1 is required for the recruitment of RAR&x03B1;/TFIIH complexes to response elements and subsequently for RAR&x03B1; target genes activation. Cancer cells characterized by a deregulated p38MAPK/MSK1 pathway, do not respond to RA, outlining the essential contribution of the RA-triggered phosphorylation cascade in RA signaling.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bruck, N., Vitoux, D., Ferry, C. et al. A coordinated phosphorylation cascade initiated by MSK1 directs RAR alpha recruitment to target gene promoters. Nat Prec (2008). https://doi.org/10.1038/npre.2008.2107.1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/npre.2008.2107.1