Abstract
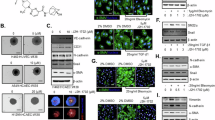
Intracellular glucocorticoid reactivation is catalyzed by 11[beta]-hydroxysteroid dehydrogenase 1 (11[beta]-HSD1), which functions predominantly as a reductase in cells expressing hexose-6-phosphate dehydrogenase (H6PDH). We recently showed that the ratios of cortisone to cortisol and 7-keto- to 7-hydroxy-neurosteroids are regulated by 11[beta]-HSD1 and very much depend on co-expression with H6PDH, providing cosubstrate NADPH. Here, we investigated the impact of H6PDH on the modulation of 11[beta]-HSD1-dependent inter-conversion of cortisone and cortisol by inhibitors and alternative substrates. Using HEK-293 cells expressing 11[beta]-HSD1 or co-expressing 11[beta]-HSD1 and H6PDH, we observed significant differences of 11[beta]-HSD1 inhibition by natural and pharmaceutical compounds as well as endogenous hormone metabolites. Furthermore, we show potent and dose-dependent inhibition of 11[beta]-HSD1 by 7-keto-DHEA in differentiated human THP-1 macrophages and in HEK-293 cells over-expressing 11[beta]-HSD1 with or without H6PDH. In contrast, 7-ketocholesterol (7-KC) did not inhibit 11[beta]-HSD1 in HEK-293 cells, even in the presence of H6PDH, but inhibited 11[beta]-HSD1 reductase activity in differentiated THP-1 macrophages (IC~50~ = 8.1 +/- 0.9 [mu]M). 7-keto-DHEA but not 7-KC inhibited 11[beta]-HSD1 in HEK-293 cell lysates. In conclusion, cellular factors such as H6PDH can significantly modulate the effect of inhibitors and alternative 7-oxygenated substrates on intracellular glucocorticoid availability.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balázs, Z., Nashev, L., Chandsawangbhuwana, C. et al. Hexose-6-phosphate dehydrogenase modulates the effect of inhibitors and alternative substrates of 11[beta]-hydroxysteroid dehydrogenase 1. Nat Prec (2008). https://doi.org/10.1038/npre.2008.2430.1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/npre.2008.2430.1
Keywords
This article is cited by
-
7-oxo-DHEA enhances impaired M. tuberculosis-specific T cell responses during HIV-TB coinfection
Journal of Biomedical Science (2020)