Abstract
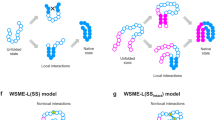
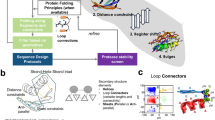
It has long since been a mystery why most proteins fold within a flash of time into particular structures out of astronomically large numbers of possible conformations. Even more confusing is that protein folding in vivo is played out in rich solution containing various organic and non-organic, big and small molecules and ions which would potentially bind the protein molecules and prevent them folding. A possible answer to these mysteries might be, "Nature have favoured such proteins that quickly fold in rich solution through natural selection". Then what mechanism of folding has been favoured? Here I show how to decipher protein sequences to reveal the folding mechanism. The entropic landscape of a protein sequence tells which region of the sequence sets out to fold first which next and last. Each step of the folding procedure is programmed in the sequence. This make it clear why proteins fold quickly and escape from surrounding molecules and ions. The folding pathways represented by the entropic landscape agree with the pathways experimentally proposed. Besides, the simulation of protein folding scheduled by the entropic landscape generates native-like conformations, where the lower the entropy of a sequential region is the earlier its conformation is optimized in terms of energy minimization. The attempt to simulate protein folding gives further insights into the folding mechanism.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Onizuka, K. The Entropic Landscape of proteins revealing protein folding mechanism. Nat Prec (2008). https://doi.org/10.1038/npre.2008.2719.1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/npre.2008.2719.1