Abstract
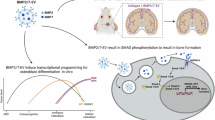
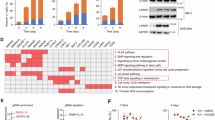
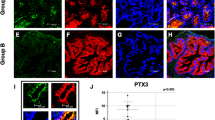
Phosphatase homologue of tensin (PTEN) is the key endogenous inhibitor of phosphoinositide signaling and is the most commonly mutated gene in human prostate cancer. The bone morphogenetic proteins (BMPs) are secreted developmental signaling molecules known to promote differentiation in the prostate. BMP ligands have been shown to inhibit prostate cancer cell line proliferation and tumor growth and expression of BMPs, BMP ligands, receptors and signaling effectors are diminished in prostate cancer. A previous report in the colon led us to investigate the potential mechanistic relationship between PTEN and BMP signaling in prostate epithelial cells. We show here that BPM signaling positively regulates PTEN in normal and malignant prostate cells by increasing mRNA expression and stabilizing PTEN protein. Further, we show that BMP attenuates prostate cell growth at least in part through its effects on PTEN. BMP treatment did not further inhibit the growth of conditional PTEN over-expressing cells, and stable shRNA-PTEN transfectants were refractory to BMP-mediated growth inhibition. Loss-of-function of PTEN in prostate cancer cells may render them insensitive to the normal differentiating and growth-inhibitory effects of BMPs. These data are the first to identify a mechanistic linkage between BMP signaling and PTEN in normal prostate epithelial cells and to suggest coordinate dysregulation in prostate cancer.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jerde, T., Wu, Z., Theodorescu, D. et al. Regulation of Phosphatase Homologue of Tensin Protein Expression by Bone Morphogenetic Proteins in Prostate Epithelial Cells. Nat Prec (2010). https://doi.org/10.1038/npre.2010.4311.1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/npre.2010.4311.1