Abstract
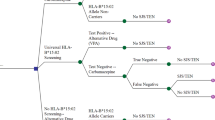
Hypersensitivity syndrome reactions (HSR) to antiepileptic drugs (AED) are associated with severe clinical cutaneous adverse reactions (SCAR).Our aims are: to assess HSRs to AEDs using the in vitro lymphocyte toxicity assay (LTA) in patients who manifested HSRs clinically, to correlate LTA results with the clinical syndrome, to correlate LTA results with the human leukocyte antigen (HLA) allele B*1502 (HLA-B*1502) positivity in a Han Chinese-Canadian population, and to determine the cytokine network in this population. HSR patients developed fever and cutaneous eruptions in the presence or absence of organ involvement within 8 weeks of exposure to carbamazepine (CBZ), phenytoin (PHY) or lamotrigine (LTG). Control patients received AEDs without presenting HSR. We investigated 10 CBZ-HSR (4 presented with Stevens-Johnson syndrome (SJS)), 24 CBZ-controls, 10 PHY-HSR (4 presented with drug-induced liver injury (DILI)), 24 PHY-controls, 6 LTG-HSR (1 SJS and 1 DILI) and 24 LTG-controls. There were 30 Han Chinese individuals (14 HSR patients and 16 controls) in our cohort. LTA toxicity greater than 12.5%&177;2.5% was considered positive. Differences among groups were determined by analysis of variance. In addition, we measured cytokine secretion in the patient sera between 1 month and 3 years after the event. All Han Chinese individuals and 30% of Caucasians were genotyped for HLA-B*1502.A perfect correlation (r=0.92) was observed between positive LTA and clinical diagnosis of DILI and SJS/toxic epidermal necrolysis (TEN). HLA-B*1502 positivity in Han Chinese is a predictor of CBZ-HSR and PHY-HSR. HLA-B*1502-negative Han Chinese receiving only CBZ or a combination of CBZ-PHY tolerated the drug(s) clinically, presenting negative CBZ-LTA and PHY-LTA. However, 3 patients presenting negative CBZ-LTA and PHY-LTA, as well as negative HLA-B*1502, showed positive LTG-LTA (38%, 28% and 25%, respectively), implying that they should not be prescribed LTG. Three patients had LTA positive to both PHY and CBZ, and 3 others had LTA positive to both PHY and LTG. Clinically, all six patients presented HSR to both drugs that they tested positive to (cross-reactivity). Patients were grouped based on the clinical presentation of their symptoms as only rash and fever or a triad that characterizes "true" HSR (rash, fever and DILI or SJS/TEN). Levels of pro-inflammatory cytokines were significantly higher in patient sera compared to control sera. More specifically, the highest levels of tumor necrosis factor (TNF)-&x03B1; was measured in patients presenting "true" HSR, as were the apoptotic markers Fas, caspase 8 activity and M30. We concluded that LTA is sensitive for DILI and SJS/TEN regardless of drug or ethnicity. HSR prediction will prevent AED-induced morbidity. In Han Chinese, HLA-B*1502 positivity is a predictor for CBZ-HSR and PHY-HSR. Its negativity does not predict a negative LTG-HSR. There is cross-reactivity between AEDs. Additionally, T-cell cytokines and chemokines control the pathogenesis of SJS/TEN and DILI, contributing to apoptotic processes in the liver and in the skin.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neuman, M., Nanau, R., Cohen, L. et al. Genetic and Immune Predictors for Hypersensitivity Syndrome to Antiepileptic Drugs. Nat Prec (2011). https://doi.org/10.1038/npre.2011.6477.1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/npre.2011.6477.1