Abstract
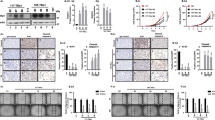
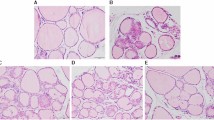
Cotransfer of TTF-1 and Pax-8 gene to tumor cells, resulting in the reexpression of iodide metabolism-associated proteins, such as sodium iodide symporter (NIS), thyroglobulin (Tg), thyroperoxidase (TPO), offers the possibility of radioiodine therapy to non-iodide-concentrating tumor because the expression of iodide metabolism-associated proteins in thyroid are mediated by the thyroid transcription factors TTF-1 and Pax-8. The human TTF-1 and Pax-8 gene were transducted into the human thyroid carcinoma (K1 and F133) cells by the recombinant adenovirus, AdTTF-1 and AdPax-8. Reexpression of NIS mRNA and protein, but not TPO and Tg mRNA and protein, was detected in AdTTF-1-infected F133 cells, following with increasing radioiodine uptake (6.1~7.4 times), scarcely iodide organification and rapid iodide efflux (t1/2≈8 min in vitro, t1/2≈4.7 h in vivo).In contrast, all of the reexpression of NIS, TPO and Tg mRNA and proteins in F133 cells were induced by the synergetic effect of TTF-1 and Pax-8. AdTTF-1 and AdPax-8 coinfected K1 and F133 cells could effectively accumulate radioiodine (6.6-7.5 times) and obviously retarded radioiodine retention (t1/2≈25-30 min in vitro, t1/2≈12 h in vivo) (p<0.05).Accordingly, the effect of radioiodine therapy of TTF-1 and Pax-8 cotransducted K1 and F133 cells (21-25% survival rate in vitro) was better than that of TTF-1-transducted cells(40% survival rate in vitro) (p<0.05). These results indicate that single TTF-1 gene transfer may have limited efficacy of radioiodine therapy because of rapid radioiodine efflux. The cotransduction of TTF-1 and Pax-8 gene, with resulting NIS-mediated radioiodine accumulation and TPO and Tg-mediated radioiodine organification and intracellular retention, may lead to effective radioiodine therapy of thyroid carcinoma.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mu, D., Huang, R., Ma, X. et al. Combining Transfer of TTF-1 and Pax-8 Gene: a Potential Strategy to Promote Radioiodine Therapy of Thyroid Carcinoma. Nat Prec (2011). https://doi.org/10.1038/npre.2011.6546.1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/npre.2011.6546.1