Key Points
-
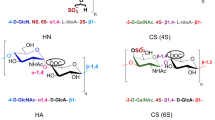
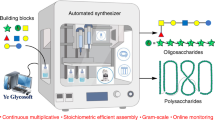
Solution-phase synthesis is a slow process due to the need for iterative coupling and deprotection steps, with purification at each step along the way. The first automated solid-phase oligosaccharide synthesizer based on a modified peptide synthesizer was introduced in 2001.
-
A number of highly successful drugs based on carbohydrates have been developed, such as acarbose, an α-glucosidase inhibitor, for the treatment of diabetes mellitus. Heparin is an even older carbohydrate-based drug that has been used since the 1940s. Much effort has been put in to preparing highly active synthetic heparin structures.
-
The presence of specific carbohydrates on the cell surface of particular types of cells opens up the possibility of creating vaccines. A hexasaccharide construct partly assembled by automated synthesis was shown to be immunogenic and to provide significant protection against malarial pathogenesis.
-
Special carbohydrate antigens, such as sialyl Lewis X and Globo-H, are known to be overexpressed on the surface of cancer cells. Patients immunized with synthetic carbohydrate-based vaccines produce antibodies that are reactive against malignant cells.
-
The presence of specific carbohydrates on the cell surface of particular cell populations presents the possibility of detecting these cells on the basis of their carbohydrate coat. This property is used in detecting even a small amount of bacteria with carbohydrate-functionalized fluorescent polymers.
Abstract
Carbohydrates present both potential and problems — their biological relevance has been recognized, but problems in procuring sugars rendered them a difficult class of compounds to handle in drug discovery efforts. The development of the first automated solid-phase oligosaccharide synthesizer and other methods to assemble defined oligosaccharides rapidly has fundamentally altered this situation. This review describes how quick access to oligosaccharides has not only contributed to biological, biochemical and biophysical investigations, but also to drug discovery. Particular focus will be placed on the development of carbohydrate-based vaccines, defined heparin oligosaccharides and aminoglycosides that have recently begun to affect drug discovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hunkapiller, T., Kaiser, R. J., Koop, B. F. & Hood, L. Large-scale and automated DNA sequence determination. Science 354, 59–67 (1991).
Caruthers, M. H. Gene synthesis machines: DNA chemistry and its uses. Science 230, 281–285 (1985). The essentials for the synthetic assembly of genes are presented.
Caruthers, M. H. Chemical synthesis of DNA and DNA analogs. Acc. Chem. Res. 24, 278–284 (1991).
Atherton, E. & Sheppard, R. C. Solid-Phase Peptide Synthesis: A Practical Approach (Oxford Univ. Press, Oxford, 1989).
Sears, P. & Wong, C. -H. Toward automated synthesis of oligosaccharides and glycoproteins. Science 291, 2344–2350 (2001).
Wong, C. -H. Enzymic and chemo-enzymic syntheses of carbohydrates. Pure Appl. Chem. 67, 1609–1616 (1995).
Plante, O. J., Palmacci, E. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001). The first automated solid-phase oligosaccharide synthesizer is presented. Linear and branched structures up to dodecasaccharides are prepared.
Seeberger, P. H. Automated carbohydrate synthesis to drive chemical glycomics. Chem. Commun. 1115–1121 (2003).
Laine, R. A. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 1012 structures for a reducing hexasaccharide: the isomer barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 4, 759–767 (1994).
Schmidt, R. R., Castro-Palomino, J. C. & Retz, O. New aspects of glycoside bond formation. Pure Appl. Chem. 71, 729–744 (1999).
Koeller, K. & Wong, C. -H. Synthesis of complex carbohydrates and glycoconjugates: enzyme-based and programmable one-pot strategies. Chem. Rev. 100, 4465–4493 (2000).
Wong, C. -H., Halcomb, R. L., Ichikawa, Y. & Kajimoto, T. Enzymes in organic synthesis: application to the problems of carbohydrate recognition. Part 1. Angew. Chem. Int. Ed. Engl. 34, 412–432 (1995).
Wong, C. -H., Halcomb, R. L., Ichikawa, Y. & Kajimoto, T. Enzymes in organic synthesis: application to the problems of carbohydrate recognition. Part 2. Angew. Chem. Int. Ed. Engl. 34, 521–546 (1995).
Chen, X. et al. Sugar nucleotide regeneration beads (superbeads): a versatile tool for the practical synthesis of oligosaccharides. J. Am. Chem. Soc. 123, 2081–2082 (2001).
Delorme, E. et al. Role of glycosylation on the secretion and biological activity of erythropoietin. Biochemistry 31, 9871–9876 (1992).
Jiang, J., Biggins, J. B. & Thorson, J. S. A general enzymatic method for the synthesis of natural and 'unnatural' UDP- and TDP-nucleotide sugars. J. Am. Chem. Soc. 122, 6803–6804 (2000).
Ye, X. -S. & Wong, C. -H. Anomeric reactivity-based one-pot oligosaccharide synthesis: a rapid route to oligosaccharide libraries. J. Org. Chem. 65, 2410–2431 (2000).
Douglas, N. L., Ley, S. V., Lucking, U. & Warriner, S. L. Tuning glycoside reactivity: new tool for efficient oligosaccharide synthesis. J. Chem. Soc., Perkin Trans. 1 51–65 (1998).
Lassaletta, J. & Schmidt, R. R. Glycosyl imidates. Part 75. Synthesis of the hexasaccharide moiety of globo H (human breast cancer) antigen. Liebigs Ann. 1417–1423 (1996). The first total synthesis of Globo-H hexasaccharide is reported.
Park, T. K. et al. Total synthesis and proof of structure of a human breast tumor (Globo-H) antigen. J. Am. Chem. Soc. 118, 11488–11500 (1996).
Bosse, F., Marcaurelle, L. A. & Seeberger, P. H. Linear synthesis of tumor-associated carbohydrate antigens Globo-H, SSEA-3, and Gb3. J. Org. Chem. 67, 6659–6670 (2002).
Takahashi, T., Adachi, M., Matsuda, A. & Doi, T. Combinatorial synthesis of trisaccharides via solution-phase one-pot glycosylation. Tetrahedron Lett. 41, 2599–2603 (2000).
Frechet, J. M. in Polymer-supported Reactions in Organic Synthesis (eds Hodge, P. & Sherrington, D. C.) 407–434 (Wiley, Chichester, 1980). Provides a review of solid-phase oligosaccharide synthesis before 1980.
Seeberger, P. H. & Haase, W. -C. Solid-phase oligosaccharide synthesis and combinatorial carbohydrate libraries. Chem. Rev. 100, 4349–4393 (2000). A recent review of solid-phase oligosaccharide assembly.
Seeberger, P. H. (ed.) Solid Support Oligosaccharide Synthesis and Combinatorial Carbohydrate Libraries (Wiley-Interscience, Chichester, 2001).
Plante, O. J., Andrade, R. B. & Seeberger, P. H. Synthesis and use of glycosyl phosphates as glycosyl donors. Org. Lett. 1, 211–214 (1999).
Schmidt, R. R. & Kinzy, W. Anomeric-oxygen activation for glycoside synthesis: the trichloroacetimidate method. Adv. Carbohydr. Chem. Biochem. 50, 21–123 (1994).
Love, K. R. & Seeberger, P. H. Automated solid-phase synthesis of protected tumor-associated antigen and blood group determinant oligosaccharides. Angew. Chem. Int. Ed. Engl. 43, 602–605 (2004). The synthesis of Lewis oligosaccharides was successfully performed on the automated oligosaccharide synthesizer starting from five different monomeric building blocks.
Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nature Rev. Drug Discov. 1, 493–502 (2002).
Manson, M. M. et al. Modulation of signal-transduction pathways by chemopreventive agents. Biochem. Soc. Trans. 28, 7–12 (2000).
Wilson, W. D., Ratmeyer, L., Zhao, M., Strekowski, L. & Boykin, D. The search for structure-specific nucleic acid-interactive drugs: effects of compound structure on RNA versus DNA interaction strength. Biochemistry 32, 4098–4104 (1993).
Geijtenbeek, T. B. H. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune response. Cell 100, 575–585 (2000).
Kansas, G. S. Selectins and their ligands: current concepts and controversies. Blood 88, 3259–3287 (1996).
Barnes-Seemann, D., Park, S. B., Koehler, A. N. & Schreiber, S. L. Expanding the functional group compatibility of small molecule microarrays: discovery of novel calmodulin ligands. Angew. Chem. Int. Ed. Engl. 42, 2478–2481 (2003).
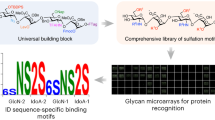
Fukui, S., Feizi, T., Galustian, C., Lawson, A. M. & Chai, W. Oligosaccharide microarrays for high throughput detection and specificity assignments of carbohydrate-protein interactions. Nature Biotechnol. 20, 1011–1017 (2002).
Hergenrother, P. J., Depew, K. M. & Schreiber, S. L. Small molecule microarrays: covalent attachment and screening of alcohol-containing small molecules on glass slides. J. Am. Chem. Soc. 122, 7849–7850 (2000).
Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 406, 775–781 (2000).
Llano-Sotelo, B., Azucena, E. F. Jr., Kotra, L. P., Mobashery, S. & Chow, C. S. Aminoglycosides modified by resistance enzymes display diminished binding to the bacterial ribosomal aminoacyl-tRNA site. Chem. Biol. 9, 455–463 (2002).
Disney, M. D., Magnet, S., Blanchard, J. S. & Seeberger, P. H. Aminoglycoside microarrays to study antibiotic resistance. Angew. Chem. Int. Ed. Engl. 43, 1591–1594 (2004).
Disney, M. D. & Seeberger, P. H. Aminoglycoside microarrays to explore interactions of antibiotics with RNAs and proteins. Chem. Eur. J. 10, 3308–3314 (2004).
Truscheit, E. et al. Chemistry and biochemistry of microbial α-glucosidase inhibitors. Angew. Chem. Int. Ed. Engl. 20, 744–761 (1981).
Capila, I. & Linhardt, R. J. Heparin–protein interactions. Angew. Chem. Int. Ed. Engl. 41, 390–412 (2002).
Tabeur, C. et al. Oligosaccharides corresponding to the regular sequence of heparin: chemical synthesis and interaction with FGF-2. Bioorg. Med. Chem. 7, 2003–2012 (1999).
Petitou, M., Casu, B. & Lindahl, U. 1976–1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie 85, 83–89 (2003).
Petitou, M. & van Boeckel, C. A. A. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew. Chem. Int. Ed. Engl. 43, 3118–3133 (2004). An excellent account of the state-of-the-art in heparin drug research.
Lee, J. -C., Lu, X. -A., Kulkarni, S. S., Wen, Y. -S. & Hung, S. -C. Synthesis of heparin oligosaccharides. J. Am. Chem. Soc. 126, 476–477 (2004).
Goldblatt, D. Recent developments in bacterial conjugate vaccines. J. Med. Microbiol. 47, 563–567 (1998).
Talbo, G. & Mann, M. Aspects of the sequencing of carbohydrates and oligonucleotides by matrix-assisted laser desorption/ionization post-source decay. Rap. Commun. Mass Spectr. 10, 100–103 (1996).
De Waard, P., Boelens, R., Vuister, G. W. & Vliegenthart, J. F. G. Structural studies by proton/carbon-13 two-dimensional and three-dimensional HMQC-NOE at natural abundance on complex carbohydrates. J. Am. Chem. Soc. 112, 3232–3234 (1990).
Sood, R. K., Fattom, A., Pavliak, V. & Naso, R. B. Capsular polysaccharide–protein conjugate vaccines. Drug Discov. Today 1, 381–387 (1996).
Tachado, S. D. et al. Signal transduction in macrophages by glycosylphosphatidylinositols of Plasmodium, Trypanosoma, and Leishmania: activation of protein tyrosine kinases and protein kinase C by inositolglycan and diacylglycerol moieties. PNAS 94, 4022–4027 (1997).
World Health Organization World Malaria situation 1990. World Health Stat. Q. 45, 257–266 (1992).
Berhe, S., Schofield, L., Schwarz, R. T. & Gerold, P. Conservation of structure among glycosylphosphatidylinositol toxins from different geographic isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 103, 273–278 (1999).
Hewitt, M. C., Snyder, D. A. & Seeberger, P. H. Rapid synthesis of a glycosylphosphatidylinositol-based malaria vaccine using automated solid-phase oligosaccharide synthesis. J. Am. Chem. Soc. 124, 13434–13436 (2002).
Schofield, L., Hewitt, M. C., Evans, K., Siomos, M. -A. & Seeberger, P. H. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 418, 785–789 (2002). This study identified a novel malaria vaccine candidate.
Mitchell, G. F. & Handman, E. The glycoconjugate derived from a Leishmania lipopolysaccharide confers parasite survival in macrophages. Parasite Immun. 8, 255–263 (1986).
Herwaldt, B. L. Leishmaniasis. Lancet 354, 1191–1199 (1999).
Turco, S. J. & Descoteaux, A. The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol. 46, 65–94 (1992).
Hewitt, M. C. & Seeberger, P. H. Solution and solid-support synthesis of a potential leishmaniasis carbohydrate vaccine. J. Org. Chem. 66, 4233–4243 (2001).
Hewitt, M. C. & Seeberger, P. H. Automated solid-phase synthesis of a branched Leishmania cap tetrasaccharide. Org. Lett. 3, 3699–3702 (2001).
Kannagi, R. et al. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J. Biol. Chem. 258, 8934–8942 (1983).
Danishefsky, S. J. & Allen, J. R. From the laboratory to the clinic: a retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew. Chem. Int. Ed. Engl. 39, 836–863 (2000).
Keding, S. J. & Danishefsky, S. J. in Carbohydrate-Based Drug Discovery Vol. 1 (ed. Wong, C.-H.) 381–406 (Wiley-VCH, Weinheim, 2003).
Menard, S., Tagliabue, E., Canevari, S., Fossati, G. & Colnaghi, M. I. Generation of monoclonal antibodies reacting with normal and cancer cells of human breast. Cancer Res. 43, 1295–1300 (1983).
Livingston, P. O. Augmenting the immunogenicity of carbohydrate tumor antigens. Cancer Biol. 6, 357–366 (1995).
Zhang, S. et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int. J. Cancer 3, 42–49 (1997).
Kitov, P. I. & Bundle, D. R. Thermodynamic models of the multivalency effect. Carbohydrate-Based Drug Discovery 2, 541–574 (2003).
Karlsson, K. A. Bacterium-host protein carbohydrate interactions and pathogenicity. Biochem. Soc. Trans. 27, 471–474 (1999).
Karlsson, K. A. Pathogen-host protein–carbohydrate interactions as the basis of important infections. Adv. Exp. Med. Biol. 491, 431–443 (2001).
Mammen, M., Choi, S. -K. & Whitesides, G. M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. Engl. 37, 2745–2794 (1998).
Disney, M. D., Zheng, J., Swager, T. M. & Seeberger, P. H. Detection of bacteria with carbohydrate-functionalized fluorescent polymer. J. Am. Chem. Soc. 126, 13343–13346 (2004).
Disney, M. D. & Seeberger, P. H. The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem. Biol. 11, 1701–1707 (2004).
Garegg, P. J. Thioglycosides as glycosyl donors in oligosaccharide synthesis. Adv. Carbohydr. Chem. Biochem. 52, 179–205 (1997).
Kahne, D., Walker, S., Cheng, Y. & van Engen, D. Glycosylation of unreactive substrates. J. Am. Chem. Soc. 111, 6881–6882 (1989).
Koenigs, W. & Knorr, E. Derivatives of grape sugar and galactose. Chem. Ber. 34, 957–981 (1901).
Mukaiyama, T., Murai, Y. & Shoda, S. An efficient method for glucosylation of hydroxy compounds using glucopyranosyl fluoride. Chem. Lett. 431–432 (1981).
Kondo, H. et al. Glycosyl phosphites as glycosylation reagents: scope and mechanism. J. Org. Chem. 59, 864–877 (1994).
Plante, O. J., Palmacci, E. R., Andrade, R. B. & Seeberger, P. H. Oligosaccharide synthesis with glycosyl phosphate and dithiophosphate triesters as glycosylating agents. J. Am. Chem. Soc. 123, 9545–9554 (2001).
Fraser-Reid, B., Konradsson, P., Mootoo, D. R. & Udodong, U. Direct elaboration of pent-4-enyl glycosides into disaccharides. J. Chem. Soc. Chem. Commun. 823–825 (1988).
Seeberger, P. H., Bilodeau, M. T. & Danishefsky, S. Synthesis of biologically important oligosaccharides and other glycoconjugates by the glycal assembly method. J. Aldrichimica Acta 30, 75–92 (1997).
de Paz, J. -L. et al. The activation of fibroblast growth factors by heparin: synthesis, structure, and biological activity of heparin-like oligosaccharides. ChemBioChem 2, 673–685 (2001).
Prabhu, A., Venot, A. & Boons, G. -J. New set of orthogonal protecting groups for the modular synthesis of heparan sulfate fragments. Org. Lett. 5, 4975–4978 (2003).
Orgueira, H. A. et al. Modular synthesis of heparin oligosaccharides. Chem. Eur. J. 9, 140–169 (2003). A highly flexible modular approach for the construction of heparin-like structures is presented.
Shukla, D. et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99, 13–22 (1999).
Acknowledgements
D.B.W. thanks the Alexander von Humboldt Foundation (AvH) for a Feodor Lynen Research Fellowship and the Deutsche Forschungsgemeinschaft (DFG) for a Emmy Noether Fellowship. P.H.S. thanks the Swiss Federal Institute of Technology (ETH) and the Swiss National Science Foundation for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P.H.S. holds stock in Ancora Pharmaceuticals, a company that utilizes automated oligosaccharide synthesis in drug discovery.
Related links
Glossary
- GENOMICS AND PROTEOMICS
-
All aspects dealing with the biology of nucleic acids and proteins. Analogous, for all aspects dealing with glycobiology the term 'glycomics' was introduced.
- POLYMERASE CHAIN REACTION (PCR)
-
The purpose of this enzymatic reaction is to make a huge number of copies of a gene. This is necessary to have enough starting template for sequencing.
- PROTECTING GROUP
-
A chemical moiety that is introduced to prevent a reaction at a certain position. Protecting groups can be permanent or temporary. The former remain during the complete synthesis in the molecule, whereas the latter protect the functional group only during some steps. Protecting groups should also be removable in an easy fashion.
- ONE-POT METHOD
-
Sequence of reactions carried out in the same reaction vessel without purifying intermediate compounds.
- SOLID-PHASE CHEMISTRY
-
In solid-phase synthesis, the compounds being synthesized are attached (usually by a linker moiety) to an insoluble polymeric material (usually beads or resin), allowing the reaction products to be readily separated (for example, by filtration or centrifugation) from excess reagent, soluble reaction by-products and solvents.
- ANTIGEN
-
Antigens are macromolecules that elicit an immune response in the body. Antigens can be proteins, conjugates of lipids with proteins (lipoproteins) or polysaccharides (glycolipids).
- SELECTINS
-
A family of carbohydrate-binding proteins (lectins) expressed on activated platelets (P-selectin) and endothelial cells (P- and E-selectin).
- EPITOPE
-
A part of an antigen to which an antibody binds. Also called an antigenic determinant.
- MICROARRAY
-
A chip, prepared by attachment of biopolymers to a surface in a spatially discrete pattern. Microarrays have enabled a low-cost and high-throughput methodology to screen interactions of molecules.
- α-GLUCOSIDASE
-
Carbohydrate-splitting enzymes that catalyse the hydrolysis of α-glucosidic linkages in oligo- and polysaccharides.
- KEYHOLE LIMPET HAEMOCYANIN
-
A high-molecular-mass polymeric glycoprotein derived from the haemolymph of the giant sea mollusk Megathura crenulata and frequently used as a highly immunogenic carrier for carbohydrate antigens.
Rights and permissions
About this article
Cite this article
Seeberger, P., Werz, D. Automated synthesis of oligosaccharides as a basis for drug discovery. Nat Rev Drug Discov 4, 751–763 (2005). https://doi.org/10.1038/nrd1823
Issue date:
DOI: https://doi.org/10.1038/nrd1823
This article is cited by
-
Automated synthesis of prexasertib and derivatives enabled by continuous-flow solid-phase synthesis
Nature Chemistry (2021)
-
Synthesis of glycoconjugates utilizing the regioselectivity of a lytic polysaccharide monooxygenase
Scientific Reports (2020)
-
Towards the generalized iterative synthesis of small molecules
Nature Reviews Chemistry (2018)
-
New agents in HSC mobilization
International Journal of Hematology (2017)
-
Synthesis of Glycosylated Chrysin Derivatives Via Ester Linkers
Chemistry of Natural Compounds (2016)