Key Points
-
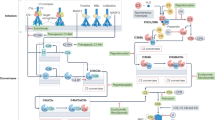
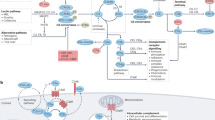
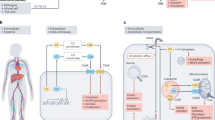
The complement system is an important part of the innate immune system that participates in tissue injury in a number of inflammatory and autoimmune diseases.
-
Polymorphisms in genes that encode complement proteins involved in activation or regulation of the system are recognized as triggers of diseases such as atypical haemolytic uraemic syndrome and age-related macular degeneration.
-
Modulation of complement activation could be beneficial in reducing tissue injury or even preventing the onset of such diseases.
-
Currently, only a few agents that are known to inhibit complement activation are approved for clinical use for narrow indications. These include an antibody to C5 (eculizumab) aimed at the treatment of paroxysmal nocturnal haemoglobinuria, Serping1 concentrate as a replacement therapy in hereditary angioedema and pooled immunoglobulin G preparations for intravenous use in some autoimmune diseases.
-
Recent literature shows that there is a growing interest in developing new and potent agents that target complement proteins and products of activation. Some examples include humanized monoclonal antibodies to complement proteins, activation products or receptors, synthetic molecules that target complement proteins or receptors, constructs of naturally occurring complement regulators and receptors modified to enhance tissue-directed activity and prolong half-life.
-
Many new agents have been tested in in vitro and in vivo animal models, generally with encouraging results. Some agents have reached clinical trials for indications such as hereditary angioedema, age-related macular degeneration, rheumatoid arthritis and psoriasis.
-
Because of a better understanding of the role of complement in many pathological states, targeting its activation holds promise as an innovative way to approach therapeutic intervention in many patients.
Abstract
The complement system is an essential component of innate immunity that has been more recently recognized as an unexpected player in various pathological states. These include age-related macular degeneration, atypical haemolytic uraemic syndrome, allergy, foetal loss, and axonal and myelin degradation after trauma. Its importance has also been recognized in physiological processes including haematopoietic stem cell homing to the bone marrow, liver regeneration and modulation of adaptive immune responses. Although the complement system has long been known to be involved in autoimmune and inflammatory diseases, few agents that target the complement system are currently approved for clinical use. However, renewed interest in modulating this system in various pathological conditions has emerged, and several agents are now in development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wagner, E. Jiang, H. & Frank, M. M. in Clinical Diagnosis and Management by Laboratory Methods (ed. Henry, J. B.) 892–913 (W. B. Saunders, Philadelphia, 2001).
Walport, M. J. Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 (2001).
Speth, C., Prodinger, W. M., Würzner, R., Stoiber, H. & Dierich, M. P. in Fundamental Immunology (ed. Paul, W. E.) 1047–1078 (Lippincott Williams & Wilkins, Philadelphia, 2008).
Zipfel, P. F., Heinen, S., Józsi, M. & Skerka, C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol. Immunol. 43, 97–106 (2006).
Frank, M. M. in The Human Complement System in Health and Disease (eds Volanakis, J. A. & Frank, M. M.) 1–8 (Marcel Dekker, New York, 1998).
Makrides, S. C. Therapeutic inhibition of the complement system. Pharmacol. Rev. 50, 59–87 (1998).
Wagner, E. & Frank, M. M. in The Human Complement System in Health and Disease (eds Volanakis, J. & Frank, M. M.) 527–546 (Marcel Dekker, New York, 1998).
Mollnes, T. E. & Kirschfink, M. Strategies of therapeutic complement inhibition. Mol. Immunol. 43, 107–121 (2006).
Cole, D. S. & Morgan, B. P. Beyond lysis: how complement influences cell fate. Clin. Sci. 104, 455–466 (2003).
Holers, V. M. The spectrum of complement alternative pathway-mediated diseases. Immunol. Rev. 223, 300–316 (2008). This review describes the alternative pathway of complement activation and its increasingly recognized role in many diseases.
Kemper, C. & Hourcade, D. E. Properdin: new roles in pattern recognition and target clearance. Mol. Immunol. 45, 4048–4056 (2008). This review presents experimental evidence of a role of properdin as a recognition molecule that directs alternative-pathway activation on microorganisms and apoptotic cells, allowing a better understanding of complement activation mechanisms.
Dommett, R. M., Klein, N. & Turner M. W. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens 68, 193–209 (2006).
Thiel, S. Complement activating soluble pattern recognition molecules with collagen-like regions, mannan-binding lectin, ficolins and associated proteins. Mol. Immunol. 44, 3875–3888 (2007).
Atkinson, J. P. & Frank, M. M. Bypassing complement: evolutionary lessons and future implications. J. Clin. Invest. 116, 1215–1218 (2006). This commentary article describes the largely unrecognized complement-bypass pathways.
Markiewski, M. M. & Lambris, J. D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 171, 715–727 (2007).
Cicardi, M., Zingale, L., Zanichelli, A., Pappalardo, E. & Cicardi, B. C1 inhibitor: molecular and clinical aspects. Springer Semin. Immun. 27, 286–298 (2005).
Chen, C.-H., Lam, C. F. & Boackle, R. J. C1 inhibitor removes the entire C1qr2s2 complex from anti-C1Q monoclonal antibodies with low binding affinities. Immunology 95, 648–654 (1998).
Chen, C.-H. & Boackle, R. J. A newly discovered function for C1 inhibitor, removal of the entire C1qr2s2 complex from immobilized human IgG subclasses. Clin. Immunol. Immunopathol. 87, 68–74 (1998).
Jiang, H., Wagner, E., Zhang, H. & Frank, M. M. Complement 1 inhibitor is a regulator of the complement alternative pathway. J. Exp. Med. 194, 1609–1616 (2001).
Nielsen, E. W. et al. Effect of supraphysiologic levels of C1-inhibitor on the classical, lectin and alternative pathways of complement. Mol. Immunol. 44, 1819–1826 (2007).
Rodriguez de Cordoba, S., Esparza-Gordillo, J., Goicoechea de Jorge, E., Lopez-Trascasa, M. & Sanchez-Corral, P. The human complement factor H: functional roles, genetic variations and disease associations. Mol. Immunol. 41, 355–367 (2004).
Jarva, H., Jokiranta, T. S., Würzner, R. & Meri, S. Complement resistance mechanisms of streptococci. Mol. Immunol. 40, 95–107 (2003).
Kim, D. D. & Song, W.-C. Membrane complement regulatory proteins. Clin. Immunol. 118, 127–136 (2006).
Wiesmann, C. et al. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature 444, 217–220 (2006).
Inal, J. M. et al. Complement C2 receptor inhibitor trispanning: a novel human complement inhibitory receptor. J. Immunol. 174, 356–366 (2005).
Carroll, M. C. The complement system in regulation of adaptive immunity. Nature Immunol. 5, 981–986 (2004).
Kemper, C. & Atkinson, J. P. T-cell regulation: with complements from innate immunity. Nature Rev. Immunol. 7, 9–18 (2007).
Monk, P. N., Scola, A.-M., Madala, P. & Fairlie, D. P. Function, structure and therapeutic potential of complement C5a receptors. Br. J. Pharmacol. 152, 429–448 (2007).
Helmy, K. Y. et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124, 915–927 (2006).
Sjöberg, A. P., Trouw, L. A. & Blom, A. M. Complement activation and inhibition: a delicate balance. Trends Immunol. 30, 83–90 (2009).
Walport M. J. Complement. Second of two parts. N. Engl. J. Med. 344, 1140–1144 (2001).
Ward, P. A. The dark side of C5a in sepsis. Nature Rev. Immunol. 4, 133–142 (2004).
Manderson, A. P., Botto, M. & Walport, M. J. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 22, 431–456 (2004).
Mitchell, D. A., Kirby, L., Paulin, S. M., Villiers, C. L. & Sim, R. B. Prion protein activates and fixes complement directly via the classical pathway: implications for the mechanisms of scrapie agent propagation in lymphoid tissue. Mol. Immunol. 44, 2997–3004 (2007).
Bonifati, D. M. & Kishore, U. Role of complement in neurodegeneration and neuroinflammation. Mol. Immunol. 44, 999–1010 (2007).
Baldwin, W. M. III, Kasper, E. K., Zachary, A. A., Wasowska, B. A. & Rodriguez, E. R. Beyond C4d: other complement-related diagnostic approaches to antibody-mediated rejection. Am. J. Transplant. 4, 311–318 (2004).
Lin, T., Zhou, W. & Sacks, S. H. The role of complement and toll-like receptors in organ transplantation. Transplant. Int. 20, 481–489 (2007).
Cugno, M., Zanichelli, A., Foieni, F., Caccia, S. & Cicardi, M. C1 inhibitor deficiency and angioedema: molecular mehanisms and clinical progress. Trends Mol. Med. 15, 69–78 (2009).
Fang, C. J., Richards, A., Liszewski, M. K., Kavanagh, D. & Atkinson, J. P. Advances in understanding of pathogenesis of aHUS and HELLP. Br. J. Haematol. 143, 336–348 (2008).
Dragon-Durey, M.-A. & Fremeaux-Bacchi, V. Atypical haemolytic uraemic syndrome and mutations in complement regulator genes. Springer Semin. Immun. 27, 359–374 (2005).
Parker, C. J. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet 373, 759–767 (2009).
Wagner, E. & Frank, M. M. in Medical Immunology (eds Parslow, T. G., Stites, D. P., Terr, A. I. & Imboden, J. B.) 341–348 (McGraw-Hill, New York, 2001).
Sullivan, K. E. & Winkelstein J. A. in Immunologic Disorders in Infants and Children (eds Stiehm, E. R., Ochs, H. D. & Winkelstein, J. A.) 652–684 (Elsevier Saunders, Philadelphia, 2004). This chapter describes the clinical impact of all of the known complement deficiency states in humans for a better understanding of the impact of inhibiting specific complement components, especially in a chronic manner.
Garred, P., Larsen, F., Madsen, H. O & Koch, C. Mannose-binding lectin deficiency — revisited. Mol. Immunol. 40, 73–84 (2003).
Eisen, D. P. & Minchinton, R. M. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 37, 1996–1505 (2003).
Jokiranta, T. S. et al. Where next with atypical hemolytic uremic syndrome? Mol. Immunol. 44, 3889–3900 (2007).
Trouw, L. A., Roos, A. & Daha, M. R. Autoantibodies to complement components. Mol. Immunol. 38, 199–206 (2001).
Agostoni, A. et al. Hereditary and acquired angioedema: proceedings of the third C1 inhibitor deficiency workshop and beyond. J. Allerg. Clin. Immunol. 114, S51–S131 (2004). This publication provides a comprehensive description of hereditary and acquired angioedema due to C1 inhibitor deficiency, including basic and clinical aspects of the disease.
Dragon-Durey, M. A. et al. Anti-factor H autoantibodies associated with atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 16, 555–563 (2005).
Rother, R. P., Rollins, S. A., Mojcik, C. F., Brodsky, R. A. & Bell, L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nature Biotech. 25, 1256–1264 (2007). This review describes the development of the C5-specific antibody currently approved for the treatment of PNH and results of experimental and clinical studies that have led to its use in a clinical setting.
Testa, L. et al. Pexelizumab in ischemic heart disease: a systematic review and meta-analysis on 15,196 patients. J. Thorac. Cardiovasc. Surg. 136, 884–893 (2008).
Locke, J. E. et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am. J. Transplant. 9, 23123–23125 (2009).
Stegall, M. et al. Prevention of acute humoral rejection with C5 inhibition [abstract]. American Society of Transplantation Winter Symposium. (Banff, Alberta, Canada, 2009).
Nürnberger, J. et al. Eculizumab for atypical hemolytic-uremic syndrome. N. Engl. J. Med. 360, 542–544 (2009).
Gruppo R. A. & Rother, R. P. Eculizumab for congenital atypical hemolytic-uremic syndrome. N. Engl. J. Med. 360, 544–546 (2009).
Dührsen, U. & Philipp, T. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood 113, 3885–3886 (2009).
Ricklin, D. & Lambris, J. D. Complement-targeted therapeutics. Nature Biotech. 25, 1265–1275 (2008). An excellent review of complement-targeting agents with an emphasis on pharmacological development.
Epstein, T. G. & Bernstein, J. A. Current and emerging management options for hereditary angioedema. Drugs 68, 2561–2573 (2008).
Caliezi, C. et al. C1-inhibitor in patients with severe sepsis and septic shock: beneficial effect on renal dysfunction. Crit. Care Med. 30, 1722–1728 (2002).
Davis, A. E. III, Mejia, P. & Lu, F. Biological activities of C1 inhibitor Mol. Immunol. 45, 4057–4063 (2008).
Struber, M. et al. C1-esterase inhibitor in graft failure after lung transplantation. Intensive Care Med. 25, 1315–1318 (1999).
Thielmann, M. et al. Administration of C1-esterase inhibitor during emergency coronary artery bypasses surgery in acute ST-elevation myocardial infarction. Eur. J. Cardiothorac. Surg. 30, 285–293 (2006).
Fattouch, K. et al. Beneficial effects of C1 esterase inhibitor in ST-elevation myocardial infarction in patients who underwent surgical reperfusion: a randomised double-blind study. Eur. J. Cardiothorac. Surg. 32, 326–332 (2007).
Baig, K. et al. Complement factor 1 inhibitor improves cardiopulmonary function in neonatal cardiopulmonary bypass. Ann. Thoracic Cardiovasc. Surg. 84, 1477–1482, (2007).
Asghar, S. S. & Pasch, M. C. Therapeutic inhibition of the complement system. Y2K update. Front. Biosci. 5, e63–e81 (2000).
Jolles, S., Sewell, W. A. C. & Misbah, S. A. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 142, 1–11 (2005).
Frank, M. M., Miletic, V. D. & Jiang, H. Immunoglobulin in the control of complement activation. Immunol. Res. 22, 137–146 (2000).
Basta, M. & Dalakas, M. C. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J. Clin. Invest. 94, 1729–1735 (1994).
Basta, M. Ambivalent effect of immunoglobulins on the complement system: activation versus inhibition. Mol. Immunol. 45, 4073–4079 (2008).
Arumugan, T. V. et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc. Natl Acad. Sci. USA 104, 14104–14109 (2008).
Weisman, H. F. et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science 249, 146–151 (1990). This paper describes the design of a complement-modulating agent based on a known complement regulator and proves its efficacy in a murine model of myocardial infarction.
Lazar, H. L. et al. Soluble human complement receptor 1 limits ischemic damage in cardiac surgery patients at high risk requiring cardiopulmonary bypass. Circulation 110 (suppl. II), 274–279 (2004).
Li, J. S., Jaggers, J. & Anderson, P. A. The use of TP10, soluble complement receptor 1, in cardiopulmonary bypass. Expert Rev. Cardiovasc. Ther. 4, 649–654 (2006).
Mocco, J. et al. Preclinical evaluation of the neuroprotective effect of soluble complement receptor type 1 in a nonhuman primate model of reperfused stroke. J. Neurosurg. 105, 595–601 (2006).
Yazdanbakhsh, K. Development of complement therapeutics for inhibition of immune-mediated red cell destruction. Transfusion 45, S122–S129 (2005).
Patel, H., Smith, A. G., Sacks, S. H. & Zhou, W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J. Am. Soc. Nephrol. 17, 1102–1111 (2006).
Williams, A. S., Linton, S. M. & Morgan B. P. Coupling complement regulators to immunoglobulin domains generates effective anti-complement reagents with extended half-life in vivo. Clin. Exp. Immunol. 129, 198–207 (2002).
Leinhase, I. et al. Pharmacological complement inhibition at the C3 convertase level promotes neuronal survival, neuroprotective intracerebral gene expression, and neurological outcome after traumatic brain injury. Exp. Neurol. 199, 454–464 (2006).
Hepburn, N. J. et al. Prevention of experimental autoimmune myasthenia gravis by rat Crry-Ig: a model agent for long-term complement inhibition in vivo. Mol. Immunol. 45, 395–405 (2008).
Huang, Y., Qiao, F., Atkinson, C., Holers, V. M. & Tomlinson, S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J. Immunol. 181, 8068–8076 (2008).
Katschke, K. Jr et al. A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J. Exp. Med. 204, 1319–1325 (2007).
Song, H. et al. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J. Clin. Invest. 111, 1875–1885 (2003).
Atkinson, C., Qiao, F., Song, H., Gilkeson, G. S. & Tomlinson, S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J. Immunol. 180, 1231–1238 (2008).
Bergmann-Leitner, E. S., Leitner, W. W. & Tsokos, G. C. Complement 3d: from molecular adjuvant to target of immune escape mechanisms. Clin. Immunol. 121, 177–185 (2006).
Bergmann-Leitner, E. S. et al. C3d-defined complement receptor-binding peptide p28 conjugated to circumsporozoite protein provides protection against Plasmodium berghei. Vaccine 25, 7732–7736 (2007).
Whipple, E. C. et al. Low doses of antigen coupled to anti-CR2 mAbs induce rapid and enduring IgG immune responses in mice and cynomolgous monkeys. Mol. Immunol. 44, 377–388 (2007).
Sprong, T. et al. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 102, 3702–3710 (2003).
Holmer, M. Genentech makes its first ever acquisition. Nature Biotech. 25, 4–5 (2007).
Thurman, J. M. et al. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 17, 707–715 (2006).
Leinhase, I. et al. Inhibition of the alternative complement activation pathway in traumatic brain injury by a monoclonal anti-factor B antibody: a randomized placebo-controlled study in mice. J. Neuroinflamm. 4, 13–25 (2007).
Taube, C. et al. Factor B of the alternative complement pathway regulates development of airway hyperresponsiveness and inflammation. Proc. Natl Acad. Sci. USA 103, 8084–8089 (2003).
Katschke, K. J. et al. Structural and functional analysis of a C3b-specific antibody that selectively inhibits the alternative pathway of complement. J. Biol. Chem. 284, 10473–10479 (2009).
Biesecker, G., Dihel, L., Enney, K. & Bendele, R. A. Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology 42, 219–230 (1999).
Holland, M. C., Morikis, D. & Lambris, J. D. Synthetic small-molecule complement inhibitors. Curr. Opin. Investig. Drugs 5, 1164–1173 (2004).
Janssen, B. J., Halff, E. F., Lambris, J. D. & Gros, P. Structure of compstatin in complex with complement component C3c reveals a new mechanism of complement inhibition, J. Biol. Chem. 282, 29241–29247 (2007).
Köhl, J. Drug evaluation: the C5a receptor antagonist PMX-53. Curr. Opin. Mol. Ther. 8, 529–538 (2006).
Vergunst, C. E. et al. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatol. 46, 1773–1778 (2007).
Marom, Z., Shelhamer, J., Berger, M., Frank, M. M. & Kaliner, M. Anaphylatoxin C3a enhances mucous glycoprotein release from human airways in vitro. J. Exp. Med. 161, 657–668 (1985).
Drouin, S. M., Corry, D. B., Hollman, T. J., Kildsgaard, J. & Wetsel, R. A. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J. Immunol. 169, 5926–5933 (2002).
Drouin, S. M., Corry, D. B., Kildsgaard, J. & Wetsel, R. A. Cutting edge: the absence of C3 demonstrates a role for complement in Th2 effector functions in a murine model of pulmonary allergy. J. Immunol. 67, 4141–4144 (2001).
Wills-Karp, M. Complement activation pathways. A bridge between innate and adaptive immune responses in asthma. Proc. Am. Thorac. Soc. 4, 247–251 (2007). This review describes the experimental evidence of the dual role of complement in asthma through anaphylatoxins C3a and C5a.
Mathieu, M.-C. et al. The C3a receptor antagonist SB 290157 has agonist activity. Immunol. Lett. 100, 139–145 (2005).
Ratajczak, J. et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood 103, 2071–2078 (2004).
von Zabern, I. in Activators and Inhibitors of Complement (ed. Sim, R. B.) 127–135 (Kluwer Academic Publishers., Dordrecht, 1993).
Younger, J. G. et al. Systemic and lung physiological changes in rats after intravascular activation of complement. J. Appl. Physiol. 90, 2289–2295 (2001).
Fritzinger, D. C. et al. Functional characterization of human C3/cobra venom factor hybrid proteins for therapeutic complement depletion. Mol. Immunol. 33, 105–116 (2009).
Lambris, J. D., Ricklin, D. & Geisbrecht, B. V. Complement evasion by human pathogens. Nature Rev. Microbiol. 6, 132–142 (2008). This review provides detailed information on the mechanisms used by microorganisms to avoid complement-mediated elimination that could be exploited as complement therapeutics.
Jongerius, I. et al. Staphylococcal complement evasion by various convertase-blocking molecules. J. Exp. Med. 204, 2461–2471 (2007).
Thorgersen, E. B. et al. Inhibition of complement and CD14 attenuates the Escherichia coli-induced inflammatory response in porcine whole blood. Infect. Immun. 77, 725–732 (2008).
Liszewski, M. K. et al. Smallpox inhibitor of complement enzymes (SPICE): regulation of complement activation on cells and mechanisms of its cellular attachment. J. Immunol. 181, 4199–4207 (2009). This recent article provides an improved understanding of the mechanism of action of complement inhibitors expressed by smallpox viruses, which is invaluable to the design of novel complement-targeting agents.
Osofsky, S. G., Thompson, B. H., Lint, T. F. & Gewurz, H. Hereditary deficiency of the third component of complement in a child with fever, skin rash, and arthralgias: response to transfusion of whole blood. J. Pediatr. 90, 180–186 (1977).
Valdimarsson, H. Infusion of plasma-derived mannan-binding lectin (MBL) into MBL-deficient humans. Biochem. Soc. Trans. 31, 768–769 (2003).
Petersen, K. A. et al. Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan-binding lectin. J. Clin. Immunol. 26, 465–475 (2006).
Casanova, J.-L. & Abel, L. Human mannose-binding lectin in immunity: friend, foe, or both? J. Exp. Med. 199, 1295–1299 (2004).
Bureeva, S., Andia-Pravdivy, J. & Kaplun, A. Drug design using the example of the complement system inhibitor's development. Drugs Discov. Today 10, 1535–1542 (2005).
Hamilton, S. R. et al. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science 313, 1441–1443 (2006). This study shows that the pichia yeast system can be used to massively produce glycoproteins bearing terminal sugars as expressed in humans, possibly paving the way to large-scale, cost-efficient production of recombinant human complement proteins.
Beinrohr, L., Dobó, J., Zavódszky, P. & Gál, P. C1, MBL-MASPs and C1 inhibitor: novel approaches for targeting complement-mediated inflammation. Trends Mol. Med. 14, 511–521 (2008).
Trapp, R. G., Fletcher, M., Forristal, J. & West, C. D. C4 binding protein deficiency in a patient with atypical Behcet's disease. J. Rheumatol. 14, 135–138 (1987).
Pickering, M. C. & Cook, H. T. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin. Exp. Immunol. 151, 210–230 (2008).
Reis, E. S., Falcao, D. A. & Isaac, L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand. J. Immunol. 63, 155–168 (2006).
Herbert, A. et al. Structure shows glycosaminoglycan- and protein-recognition site in factor H is perturbed by age-related macular degeneration-linked SNP. J. Biol. Chem. 282, 18960–18968 (2007).
Kavanagh, D, Richards, A. & Atkinson, J. P. Complement regulatory genes and haemolytic uremic syndromes. Annu. Rev. Med. 59, 293–309 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
http://www.ncbi.nlm.nih.gov/gene
OMIM
http://www.ncbi.nlm.nih.gov/omim
Glossary
- Age-related macular degeneration
-
The principal cause of blindness in the elderly in industrialized countries. It is characterized by a loss of retinal pigment epithelium and photoreceptors, and deposits of proteins and lipids (drusen) or neovascularization.
- Innate immune system
-
Innate immunity is said to be nonspecific, but many of its components, including complement, recognize pathogen associated molecular patterns (PAMPs) for initiation of a defence reaction.
- Anaphylatoxins
-
Small peptides derived from C3 and C5 cleavage that promote inflammation by inducing leukocyte chemotaxis and increasing vascular permeability, among other activities.
- Kinin pathway
-
Triggers the generation of bradykinin, a potent vasoactive peptide, upon activation of the contact system of the coagulation cascade.
- Decay-accelerating activity
-
Shortening of the half-lives of convertases of the complement activation cascade by CR1 and DAF by accelerated dissociation of their components.
- Immunological memory
-
The ability of the immune system to recognize and respond rapidly and vigorously to a specific antigen with which it has previously had contact.
- Opsonization
-
Coating of an organism with complement fragments that leads to recognition by specific receptors on phagocytes and elimination by phagocytosis.
- Systemic lupus erythematosus
-
A prototypical autoimmune disease characterized by the presence of antibodies directed against intracellular antigens (for example, DNA) that form immune complexes causing inflammation at sites of deposition such as the skin, joints and kidneys.
- Ischaemia–reperfusion injury
-
A condition that arises upon restoration of blood flow in an organ deprived of oxygen supply.
- Hereditary angioedema
-
Condition that is manifested by recurrent episodes of skin swelling and abdominal pain; may be fatal if swelling affects the larynx.
- Atypical haemolytic uraemic syndrome
-
Renal pathology manifested by a triad of microangiopathic haemolytic anaemia, thrombocytopaenia and acute renal failure. It can be sporadic or familial.
- Hyperacute rejection
-
This occurs within minutes of revascularization of the organ graft and is caused by complement-activating pre-formed antibodies reacting against endothelial cells. By contrast, acute rejection occurs within the first weeks following transplantation because of elicited antibodies.
- Cell homing
-
The process by which haematopoietic stem and progenitor cells are contained within the bone marrow and travel to this site when transplanted.
- Regulator of complement activation protein family
-
These include factor H, C4BP, DAF, MCP, CR1 and CR2. They share a common structure consisting of a number of repetitive units of 60 amino acids called short consensus repeats, or complement control protein modules, which contain binding sites for activated complement proteins.
Rights and permissions
About this article
Cite this article
Wagner, E., Frank, M. Therapeutic potential of complement modulation. Nat Rev Drug Discov 9, 43–56 (2010). https://doi.org/10.1038/nrd3011
Published:
Issue date:
DOI: https://doi.org/10.1038/nrd3011
This article is cited by
-
Induced neural stem cell grafts exert neuroprotection through an interaction between Crry and Akt in a mouse model of closed head injury
Stem Cell Research & Therapy (2021)
-
The involvement of C5a in the progression of experimental arthritis with Porphyromonas gingivalis infection in SKG mice
Arthritis Research & Therapy (2018)
-
Induced neural stem cell-derived astrocytes modulate complement activation and mediate neuroprotection following closed head injury
Cell Death & Disease (2018)
-
Pharmacologic Complement Inhibition in Clinical Transplantation
Current Transplantation Reports (2017)
-
Bothrops snake venoms and their isolated toxins, an L-amino acid oxidase and a serine protease, modulate human complement system pathways
Journal of Venomous Animals and Toxins including Tropical Diseases (2015)