Key Points
-
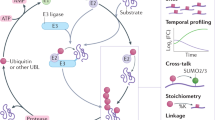
Ubiquitin is a highly conserved 76 amino-acid protein that covalently attaches to protein substrates targeted for degradation by the 26S proteasome. The coordinated effort of a series of enzymes, including an activating enzyme (E1), a conjugating enzyme (E2) and a ligase (E3), uses ATP to ultimately form an isopeptide bond between ubiquitin and a substrate.
-
Another class of enzymes called deubiquitylating enzymes (DUBs) deconstruct these linkages and also have an essential role in ubiquitin function. In addition, ubiquitin-like proteins (UBLs), including NEDD8, SUMO and ISG15, share a characteristic three-dimensional fold with ubiquitin but have their own dedicated enzyme cascades and distinct (although sometimes overlapping) biological functions.
-
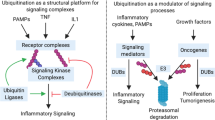
The ubiquitin–proteasome system (UPS) and UBL conjugation pathways have important roles in various human diseases, including numerous types of cancer, cardiovascular disease, viral diseases and neurodegenerative disorders. The proteasome inhibitor bortezomib (Velcade; Millennium Pharmaceuticals) is the first clinically validated drug to target the UPS and is approved for the treatment of multiple myeloma. This suggests that other diseases may conceivably be targeted by modulating components of the UPS and UBL conjugation pathways using small-molecule inhibitors.
-
A significant hurdle to identifying drug-like inhibitors of enzyme targets within the UPS and UBL conjugation pathways is the limited understanding of the molecular mechanisms and biological consequences of UBL conjugation.
-
Here, we provide an overview of the enzyme classes in the UPS and UBL pathways that are potential therapeutic targets, and highlight considerations that are important for drug discovery. We also discuss the progress in the development of small-molecule inhibitors, and review developments in understanding of the role of the components of the UPS and the UBL pathways in disease and their potential for therapeutic intervention.
Abstract
The ubiquitin–proteasome system (UPS) and ubiquitin-like protein (UBL) conjugation pathways are integral to cellular protein homeostasis. The growing recognition of the fundamental importance of these pathways to normal cell function and in disease has prompted an in-depth search for small-molecule inhibitors that selectively block the function of these pathways. However, our limited understanding of the molecular mechanisms and biological consequences of UBL conjugation is a significant hurdle to identifying drug-like inhibitors of enzyme targets within these pathways. Here, we highlight recent advances in understanding the role of some of these enzymes and how these new insights may be the key to developing novel therapeutics for diseases including immuno-inflammatory disorders, cancer, infectious diseases, cardiovascular disease and neurodegenerative disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ciechanover, A, & Schwartz, A. L. The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death. Proc. Natl Acad. Sci. USA 95, 2727–2730 (1998).
Hoeller, D, & Dikic, I. Targeting the ubiquitin system in cancer therapy. Nature 458, 438–444 (2009).
Lehman, N. L. The ubiquitin proteasome system in neuropathology. Acta Neuropathol. 118, 329–347 (2009).
Corn, P. G. Role of the ubiquitin proteasome system in renal cell carcinoma. BMC Biochem. 8, S4 (2007).
Herrmann, J., Ciechanover, A., Lerman, L. O. & Lerman, A. The ubiquitin–proteasome system in cardiovascular diseases-a hypothesis extended. Cardiovasc. Res. 61, 11–21 (2004).
Petroski, M. D. The ubiquitin system, disease, and drug discovery. BMC Biochem. 9, S7 (2008).
Jin, J., Li, X., Gygi, S. P. & Harper, J. W. Dual E1 activation systems for ubiquitin differentially regulate E2 enzyme charging. Nature 447, 1135–1138 (2007).
Chiu, Y. H., Sun, Q. & Chen, Z. J. E1-L2 activates both ubiquitin and FAT10. Mol. Cell 27, 1014–1023 (2007).
Haglund, K., Di Fiore, P. P. & Dikic, I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem. Sci. 28, 598–603 (2003).
Haglund, K., & Dikic, I. Ubiquitylation and cell signaling. EMBO J. 24, 3353–3359 (2005).
Mukhopadhyay, D. & Riezman, H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315, 201–205 (2007).
Komander, D., Clague, M. J. & Urbe, S. Breaking the chains: structure and function of the deubiquitinases. Nature Rev. Mol. Cell Biol. 10, 550–563 (2009).
Sun, Y. Targeting E3 ubiquitin ligases for cancer therapy. Cancer Biol. Ther. 2, 623–629 (2003).
Singhal, S., Taylor, M. C. & Baker, R. T. Deubiquitylating enzymes and disease. BMC Biochem. 9, S3 (2008).
Schulman, B. A. & Harper, J. W. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nature Rev. Mol. Cell Biol. 10, 319–331 (2009).
Bohnsack, R. N. & Haas, A. L. Conservation in the mechanism of NEDD8 activation by the human AppBp1–Uba3 heterodimer. J. Biol. Chem. 278, 26823–26830 (2003).
Huang, D. T. et al. A unique E1–E2 interaction required for optimal conjugation of the ubiquitin-like protein NEDD8. Nature Struct. Mol. Biol. 11, 927–935 (2004).
Ye, Y. & Rape, M. Building ubiquitin chains: E2 enzymes at work. Nature Rev. Mol. Cell Biol. 10, 755–764 (2009).
Hochstrasser, M. Lingering mysteries of ubiquitin-chain assembly. Cell 124, 27–34 (2006).
Komander, D. et al. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 10, 466–473 (2009). The structure of K63-linked and linear ubiquitin dimers, and the specificity of various DUBs and UBDs, highlight the specificity within the ubiquitin system.
David, Y., Ziv, T., Admon, A. & Navon, A. The E2 ubiquitin conjugating enzymes direct polyubiquitination to preferred lysines. J. Biol. Chem. 285, 8595–8604 (2010). Uses a systematic approach to evaluate the role of E2 enzymes in determining the topology of the different polyubiquitin chains. The analysis shows that E2s are capable of directing chain formation to distinct subsets of ubiquitin lysines independently of an E3 enzyme.
van Wijk, S. J., & Timmers, H. T. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. 24, 981–993 (2010).
Petroski, M. D. & Deshaies, R. J. Function and regulation of cullin-RING ubiquitin ligases. Nature Rev. Mol. Cell Biol. 6, 9–20 (2005).
Aghajan, M. et al. Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nature Biotech. 28, 738–742 (2010).
Orlicky, S. et al. An allosteric inhibitor of substrate recognition by the SCF(Cdc4) ubiquitin ligase. Nature Biotech. 28, 733–737 (2010).
Ito, T. et al. Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350 (2010).
Sowa, M. E., Bennett, E. J., Gygi, S. P. & Harper, J. W. Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 (2009). This systematic investigation of DUB function uses proteomic analysis of DUBs and their associated protein complexes to identify over 750 candidate interacting proteins associated with 75 DUBs, providing the first glimpse into the global DUB interaction landscape.
Bianchi, K. & Meier, P. A tangled web of ubiquitin chains: breaking news in TNF-R1 signaling. Mol. Cell 36, 736–742 (2009).
Chen, Z. J. & Sun, L. J. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 33, 275–286 (2009).
Tokunaga, F. et al. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nature Cell Biol. 11, 123–132 (2009). NF-κB is a key transcription factor for inflammatory, anti-apoptotic and immune processes and is regulated by the ubiquitin pathway. The LUBAC E3 ligase complex is composed of two RING finger proteins (HOIL-1L and HOIP), forms head-to-tail-linked linear ubiquitin chains and activates NF-κB, suggesting a physiological function for this recently identified form of polyubiquitin.
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009).
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009).
Laplantine, E. et al. NEMO specifically recognizes K63-linked poly-ubiquitin chains through a new bipartite ubiquitin-binding domain. EMBO J. 28, 2885–2895 (2009).
Xia, Z. P. et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461, 114–119 (2009).
Vaux, D. L. & Silke, J. IAPs, RINGs and ubiquitylation. Nature Rev. Mol. Cell Biol. 6, 287–297 (2005).
Chen, A. et al. The HECT-type E3 ubiquitin ligase AIP2 inhibits activation-induced T-cell death by catalyzing EGR2 ubiquitination. Mol. Cell. Biol. 29, 5348–5356 (2009).
Komander, D. et al. The structure of the CYLD USP domain explains its specificity for Lys63-linked polyubiquitin and reveals a B box module. Mol. Cell 29, 451–464 (2008).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Shembade, N., Ma, A. & Harhaj, E. W. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010).
Vereecke, L., Beyaert, R. & van Loo, G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 30, 383–391 (2009).
Hymowitz, S. G. & Wertz, I. E. A20: from ubiquitin editing to tumour suppression. Nature Rev. Cancer 10, 332–341 (2010).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Degterev, A. et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nature Chem. Biol. 4, 313–321 (2008).
Declercq, W., Vanden, B. T. & Vandenabeele, P. RIP kinases at the crossroads of cell death and survival. Cell 138, 229–232 (2009).
Xu, J. et al. NF-κB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 20, 7–17 (2009).
Dreger, H. et al. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 83, 354–361 (2009).
Tsukamoto, O., Minamino, T. & Kitakaze, M. Functional alterations of cardiac proteasomes under physiological and pathological conditions. Cardiovasc. Res. 85, 339–346 (2010).
Yu, X. & Kem, D. C. Proteasome inhibition during myocardial infarction. Cardiovasc. Res. 85, 312–320 (2010).
Sohns, W., van Veen, T. A. & van der Heyden, M. A. Regulatory roles of the ubiquitin-proteasome system in cardiomyocyte apoptosis. Curr. Mol. Med. 10, 1–13 (2010).
Enrico, O. et al. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br. J. Haematol. 138, 396–397 (2007).
Luo, H., Wong J. & Wong, B. Protein degradation systems in viral myocarditis leading to dilated cardiomyopathy. Cardiovasc. Res. 85, 347–356 (2010).
Chen, P. C. et al. The proteasome-associated deubiquitinating enzyme Usp14 is essential for the maintenance of synaptic ubiquitin levels and the development of neuromuscular junctions. J. Neurosci. 29, 10909–10919 (2009).
Haas, K. F. & Broadie, K. Roles of ubiquitination at the synapse. Biochim. Biophys. Acta 1779, 495–506 (2008).
Dawson, T. M. & Dawson, V. L. The role of parkin in familial and sporadic Parkinson's disease. Mov. Disord. 25, S32–S39 (2010).
Dawson, T. M. Parkin and defective ubiquitination in Parkinson's disease. J. Neural Transm. Suppl. 209–213 (2006).
Bedford, L. et al. Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J. Neurosci. 28, 8189–8198 (2008).
Bedford, L. et al. Is malfunction of the ubiquitin proteasome system the primary cause of alpha-synucleinopathies and other chronic human neurodegenerative disease? Biochim. Biophys. Acta 1782, 683–690 (2008).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Komatsu, M. et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 (2006).
Bedford, L. et al. The UPS and autophagy in chronic neurodegenerative disease: six of one and half a dozen of the other — or not? Autophagy 5, 224–227 (2009).
Matsuda, N. & Tanaka, K. Does impairment of the ubiquitin-proteasome system or the autophagy-lysosome pathway predispose individuals to neurodegenerative disorders such as Parkinson's disease? J. Alzheimers Dis. 19, 1–9 (2010).
Raiborg, C. & Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452 (2009).
Rusten, T. E., Filimonenko, M., Rodahl, L. M., Stenmark, H. & Simonsen, A. ESCRTing autophagic clearance of aggregating proteins. Autophagy 4, 233–236 (2007).
Hetz, C. et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 23, 2294–2306 (2009).
Paine, S. et al. Immunoreactivity to Lys63-linked polyubiquitin is a feature of neurodegeneration. Neurosci. Lett. 460, 205–208 (2009).
McGowan, E., Eriksen, J. & Hutton, M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 22, 281–289 (2006).
Melrose, H. L., Lincoln, S. J., Tyndall, G. M. & Farrer, M. J. Parkinson's disease: a rethink of rodent models. Exp. Brain Res. 173, 196–204 (2006).
Lee, B. H. et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467, 179–184 (2010). USP14, a proteasome-associated DUB, is shown to inhibit the degradation of ubiquitin–protein conjugates by trimming ubiquitin chains. A small-molecule inhibitor of USP14's DUB activity enhances the degradation of proteasome substrates in cells, including some implicated in neurodegenerative disease, suggesting a potential strategy to reduce the levels of aberrant proteins in cells under proteotoxic stress.
Rubinsztein, D. C., Gestwicki, J. E., Murphy, L. O. & Klionsky, D. J. Potential therapeutic applications of autophagy. Nature Rev. Drug Discov. 6, 304–312 (2007).
Maruyama, H. et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465, 223–226 (2010).
Zhu, G., Wu, C. J. Zhao, Y. & Ashwell, J. D. Optineurin negatively regulates TNFalpha- induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr. Biol. 17, 1438–1443 (2007).
Orlowski, R. Z. & Kuhn, D. J. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin. Cancer Res. 14, 1649–1657 (2008).
Lesniak, M. S. & Brem, H. Targeted therapy for brain tumours. Nature Rev. Drug Discov. 3, 499–508 (2004).
Scheffner, M., Werness, B. A., Huibregtse, J. M., Levine, A. J. & Howley, P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63, 1129–1136 (1990).
Hingamp, P. M., Arnold, J. E., Mayer, R. J. & Dixon, L. K. A ubiquitin conjugating enzyme encoded by African swine fever virus. EMBO J. 11, 361–366 (1992).
Zhu, Y. et al. Structure of a Shigella effector reveals a new class of ubiquitin ligases. Nature Struct. Mol. Biol. 15, 1302–1308 (2008).
Edelmann, M. J. & Kessler, B. M. Ubiquitin and ubiquitin-like specific proteases targeted by infectious pathogens: emerging patterns and molecular principles. Biochim. Biophys. Acta 1782, 809–816 (2008).
Chang, T. H. et al. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 5, e1000493 (2009).
Morita, E. & Sundquist, W. I. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20, 395–425 (2004).
Kyei, G. B. et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J. Cell Biol. 186, 255–268 (2009).
Harty, R. N., Pitha, P. M. & Okumura, A. Antiviral activity of innate immune protein ISG15. J. Innate Immunol. 1, 397–404 (2009).
Zheng, Y. T. et al. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 183, 5909–5916 (2009).
Thurston, T. L., Ryzhakov G., Bloor S., von Muhlinen, N. & Randow, F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nature Immunol. 10, 1215–1221 (2009). NDP52, a factor previously not known to contribute to innate immunity, is shown to activate autophagy against invading bacteria by collaborating with the ubiquitin system.
Yoshikawa, Y. et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nature Cell Biol. 11, 1233–1240 (2009).
Burns, K. E., Pearce, M. J. & Darwin, K. H. Prokaryotic ubiquitin-like protein provides a two-part degron to Mycobacterium proteasome substrates. J. Bacteriol. 192, 2933–2935 (2010).
World Health Organization. Fact sheet N. °104: Tuberculosis. WHO website [online], (2010).
Darwin, K. H., Ehrt, S., Gutierrez-Ramos, J. C., Weich, N. & Nathan, C. F. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302, 1963–1966 (2003).
Pearce, M. J., Mintseris, J., Ferreyra, J., Gygi, S. P. & Darwin, K. H. Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science 322, 1104–1107 (2008). In Mtb, Pup is specifically conjugated to proteasome substrates, revealing a bacterial system that uses small-protein modifiers to control protein stability. Pup is essential for the pathogenesis of Mtb, suggesting that the various components of the Pupylation system may present opportunities for therapeutic intervention.
Cerda-Maira, F. A. et al. Molecular analysis of the prokaryotic ubiquitin-like protein (Pup) conjugation pathway in Mycobacterium tuberculosis. Mol. Microbiol. 77, 1123–1135 (2010).
Burns, K. E. et al. “Depupylation” of prokaryotic ubiquitin-like protein from mycobacterial proteasome substrates. Mol. Cell 39, 821–827 (2010).
Wang, T. et al. Structural insights on the Mycobacterium tuberculosis proteasomal ATPase Mpa. Structure 17, 1377–1385 (2009).
Lin, G. et al. Mycobacterium tuberculosis prcBA genes encode a gated proteasome with broad oligopeptide specificity. Mol. Microbiol. 59, 1405–1416 (2006).
Hu, G. et al. Structure of the Mycobacterium tuberculosis proteasome and mechanism of inhibition by a peptidyl boronate. Mol. Microbiol. 59, 1417–1428 (2006).
Lin, G., Tsu, C., Dick, L., Zhou, X. K. & Nathan, C. Distinct specificities of Mycobacterium tuberculosis and mammalian proteasomes for N-acetyl tripeptide substrates. J. Biol. Chem. 283, 34423–34431 (2008).
Lin, G. et al. Inhibitors selective for mycobacterial versus human proteasomes. Nature 461, 621–626 (2009).
Walsh, R. C. et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation 89, 277–284 (2010). Relates the initial experience of using proteasome inhibition as first-line therapy to treat refractory antibody-mediated rejection (AMR). The results suggest that proteasome inhibitor-based combination therapy may provide a means for rapid donor-specific anti-human leukocyte antigen antibodies elimination in early acute AMR in renal transplant recipients.
Borissenko, L. & Groll, M. 20S proteasome and its inhibitors: crystallographic knowledge for drug development. Chem. Rev. 107, 687–717 (2007).
Demo, S. D. et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 67, 6383–6391 (2007).
Zhou, H. J. et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047). J. Med. Chem. 52, 3028–3038 (2009).
Roccaro, A. M. et al. Selective inhibition of chymotrypsin-like activity of the immunoproteasome and constitutive proteasome in Waldenstrom macroglobulinemia. Blood. 115 4051–4060 (2010).
Chauhan, D. et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell 8, 407–419 (2005).
Kupperman, E. et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 70, 1970–1980 (2010).
Piva, R. et al. CEP-18770: a novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomib. Blood 111, 2765–2775 (2008).
Dick, L. R. & Fleming, P. E. Building on bortezomib: second-generation proteasome inhibitors as anti-cancer therapy. Drug Discov. Today 15, 243–249 (2010).
Dorsey, B. D. et al. Discovery of a potent, selective, and orally active proteasome inhibitor for the treatment of cancer. J. Med. Chem. 51, 1068–1072 (2008).
Muchamuel, T. et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nature Med. 15, 781–787 (2009).
Kuhn, D. J. et al. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood 113, 4667–4676 (2009).
Yang, Y. et al. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 67, 9472–9481 (2007).
Xu, G. W. et al. The ubiquitin-activating enzyme E1 as a therapeutic target for the treatment of leukemia and multiple myeloma. Blood 115, 2251–2259 (2010).
Kitagaki, J. et al. Nitric oxide prodrug JS-K inhibits ubiquitin E1 and kills tumor cells retaining wild-type p53. Oncogene 28, 619–624 (2009).
Soucy, T. A. et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 (2009). The NEDD8 conjugation pathway controls the activity of the cullin-RING ligases and regulates the turnover of a large subset of proteins targeted for degradation by the proteasome. MLN4924, a selective inhibitor of the NEDD8-activating enzyme, blocks cullin-RING ligase-mediated protein turnover leading to apoptotic death in human tumour cells by the deregulation of S-phase DNA synthesis.
Soucy, T. A., Smith, P. G. & Rolfe, M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin. Cancer Res. 15, 3912–3916 (2009).
Chiba, T. & Tanaka, K. Cullin-based ubiquitin ligase and its control by NEDD8-conjugating system. Curr. Protein Pept. Sci. 5, 177–184 (2004).
Duda, D. M. et al. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell 134, 995–1006 (2008).
Traore, T. et al. Pre-clinical antitumor activity of MLN4924, an inhibitor of NEDD8-activating enzyme (NAE), in a novel primary human DLBCL xenograft model. Blood 114, 382a (2009).
Swords, R. T. et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood 115, 3796–3800 (2010).
Brownell, J. E. et al. Substrate-assisted inhibition of ubiquitin-like protein activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol. Cell 37, 102–111 (2010).
Maeda, H. et al. Ubiquitin-conjugating enzyme UBE2Q2 suppresses cell proliferation and is down-regulated in recurrent head and neck cancer. Mol. Cancer Res. 7, 1553–1562 (2009).
Duan, X., Trent, J. O. & Ye, H. Targeting the SUMO E2 conjugating enzyme Ubc9 interaction for anti-cancer drug design. Anticancer Agents Med. Chem. 9, 51–54 (2009).
Mo, Y. Y. & Moschos, S. J. Targeting Ubc9 for cancer therapy. Expert Opin. Ther. Targets 9, 1203–1216 (2005).
Zhu, S. Sachdeva, M., Wu, F., Lu, Z. & Mo, Y. Y. Ubc9 promotes breast cell invasion and metastasis in a sumoylation-independent manner. Oncogene 29, 1763–1772 (2010).
Hao, J. et al. Elevated expression of UBE2T in lung cancer tumors and cell lines. Tumour Biol. 29, 195–203 (2008).
van Ree, J. H., Jeganathan, K. B., Malureanu, L. & van Deursen, J. M. Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J. Cell Biol. 188, 83–100 (2010).
Chen, S. et al. Association of clinicopathological features with UbcH10 expression in colorectal cancer. J. Cancer Res. Clin. Oncol. 136, 419–426 (2010).
Huang, A. et al. E2-c-Cbl recognition is necessary but not sufficient for ubiquitination activity. J. Mol. Biol. 385, 507–519 (2009).
Das, R. et al. Allosteric activation of E2-RING finger-mediated ubiquitylation by a structurally defined specific E2-binding region of gp78. Mol. Cell 34, 674–685 (2009).
Wu, P. Y. et al. A conserved catalytic residue in the ubiquitin-conjugating enzyme family. EMBO J. 22, 5241–5250 (2003).
Reyes-Turcu, F. E., Ventii, K. H. & Wilkinson, K. D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 78, 363–397 (2009).
Love, K. R., Catic, A., Schlieker, C. & Ploegh, H. L. Mechanisms, biology and inhibitors of deubiquitinating enzymes. Nature Chem. Biol. 3, 697–705 (2007).
Cohn, M. A. et al. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol. Cell 28, 786–797 (2007).
Masuya, D. et al. The HAUSP gene plays an important role in non-small cell lung carcinogenesis through p53-dependent pathways. J. Pathol. 208, 724–732 (2006).
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature 424, 793–796 (2003).
Liu, Y. et al. The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell 111, 209–218 (2002).
Daviet, L. & Colland, F. Targeting ubiquitin specific proteases for drug discovery. Biochimie 90, 270–283 (2008).
Ghosh, A. K. et al. Structure-based design, synthesis, and biological evaluation of a series of novel and reversible inhibitors for the severe acute respiratory syndrome-coronavirus papain-like protease. J. Med. Chem. 52, 5228–5240 (2009).
Ratia, K. et al. A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc. Natl Acad. Sci. USA 105, 16119–16124 (2008).
Sierra, M. I., Wright, M. H. & Nash, P. D. AMSH interacts with ESCRT-0 to regulate the stability and trafficking of CXCR4. J. Biol. Chem. 285, 13990–14004 (2010). The role of the JAMM domain deubiquitinating enzyme AMSH in the regulation of the endocytic sorting and down-regulation of the chemokine receptor CXCR4 was investigated. Reversible ubiquitylation modulates the activity of the endocytic machinery, suggesting that AMSH may directly regulate endocytic adaptor protein function by specifying the fate of endocytosed receptors.
Tatsumi, K. et al. A novel type of E3 ligase for the Ufm1 conjugation system. J. Biol. Chem. 285, 5417–5427 (2010).
Wertz, I. E. & Dixit, V. M. Signalling to NF-κB: regulation by ubiquitin. Cold Spring Harb. Perspect. Biol. 2, a003350 (2010).
Gautheron, J. & Courtois, G. “Without Ub I am nothing”: NEMO as a multifunctional player in ubiquitin-mediated control of NF-κB activation. Cell. Mol. Life Sci. 67, 3101–3113 (2010).
Herrmann, J., Lerman, L. O. & Lerman, A. Ubiquitin and ubiquitin-like proteins in protein regulation. Circ. Res. 100, 1276–1291 (2007).
Hochstrasser, M. Origin and function of ubiquitin-like proteins. Nature 458, 422–429 (2009).
Zhang, Z. et al. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J. Biol. Chem. 279, 16000–16006 (2004).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Issaeva, N. et al. Small molecule RITA binds to p53, blocks p53–HDM-2 interaction and activates p53 function in tumors. Nature Med. 10, 1321–1328 (2004).
Gopal, Y. N., Chanchorn, E. & Van Dyke, M. W. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol. Cancer Ther. 8, 552–562 (2009).
Cardozo, T. & Pagano, M. Wrenches in the works: drug discovery targeting the SCF ubiquitin ligase and APC/C complexes. BMC Biochem. 8, S9 (2007).
Latres, E., Chiaur, D. S. & Pagano, M. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene 18, 849–854 (1999).
Read, M. A. et al. NEDD8 modification of cul-1 activates SCF(beta(TrCP)-dependent ubiquitination of IκBα. Mol. Cell Biol. 20, 2326–2333 (2000).
Srinivasula, S. M. & Ashwell, J. D. IAPs: what's in a name? Mol. Cell 30, 123–135 (2008).
Galban, S. & Duckett, C. S. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 17, 54–60 (2010).
Neil, J. R., Tian, M. & Schiemann, W. P. X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-β-mediated nuclear factor-κB activation during breast cancer progression. J. Biol. Chem. 284, 21209–21217 (2009).
Carter, B. Z. et al. Triptolide sensitizes AML cells to TRAIL-induced apoptosis via decrease of XIAP and p53-mediated increase of DR5. Blood 111, 3742–3750 (2008).
Carter, B. Z. et al. Simultaneous activation of p53 and inhibition of XIAP enhance the activation of apoptosis signaling pathways in AML. Blood 115, 306–314 (2010).
Cummings, J. et al. Method validation and preliminary qualification of pharmacodynamic biomarkers employed to evaluate the clinical efficacy of an antisense compound (AEG35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br. J. Cancer. 95, 42–48 (2006).
Sanduja, S., Kaza, V. & Dixon, D. A. The mRNA decay factor tristetraprolin (TTP) induces senescence in human papillomavirus-transformed cervical cancer cells by targeting E6-AP ubiquitin ligase. Aging (Albany NY). 1, 803–817 (2009).
Matsuura, T. et al. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nature Genet. 15, 74–77 (1997).
Rathmell, W. K. & Chen S. VHL inactivation in renal cell carcinoma: implications for diagnosis, prognosis and treatment. Expert Rev. Anticancer Ther. 8, 63–73 (2008).
Paltoglou, S. & Roberts, B. J. HIF-1alpha and EPAS ubiquitination mediated by the VHL tumour suppressor involves flexibility in the ubiquitination mechanism, similar to other RING E3 ligases. Oncogene 26, 604–609 (2007).
van Hagen, M., Overmeer, R. M., Abolvardi, S. S. & Vertegaal, A. C. RNF4 and VHL regulate the proteasomal degradation of SUMO-conjugated hypoxia-inducible factor-2α. Nucleic Acids Res. 38, 1922–1931 (2010).
Sufan, R. I. et al. Oxygen-independent degradation of HIF-alpha via bioengineered VHL tumour suppressor complex. EMBO Mol. Med. 1, 66–78 (2009).
Nakamura, T. & Lipton, S. A. Cell death: protein misfolding and neurodegenerative diseases. Apoptosis 14, 455–468 (2009).
Alchanati, I. et al. The E3 ubiquitin-ligase Bmi1/Ring1A controls the proteasomal degradation of Top2alpha cleavage complex — a potentially new drug target. PLoS One 4, e8104 (2009).
Adams, J. The proteasome: a suitable antineoplastic target. Nature Rev. Cancer. 4, 349–360 (2004).
Adams, J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5, 417–421 (2004).
Altun, M. et al. Effects of PS-341 on the activity and composition of proteasomes in multiple myeloma cells. Cancer Res. 65, 7896–7901 (2005).
Kuhn, D. J. et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 110, 3281–3290 (2007).
Feling, R. H. et al. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed Engl. 42, 355–357 (2003).
Acknowledgements
The authors thank S. Hill and N. Beavan of FireKite for assistance in the development of this manuscript. L.B., J.L. and R.J.M. thank the Alzheimer's Research Trust and Parkinson's UK for generous support of some of the work reported here.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Lawrence R. Dick and James E. Brownell are employees of Millennium Pharmaceuticals. Lynn Bedford, James Lowe and R. John Mayer declare no competing financial interests.
Glossary
- Ubiquitin
-
A highly conserved 76 amino-acid protein that can be reversibly attached to other proteins. Key structural features of ubiquitin include its β-grasp fold (a characteristic of all ubiquitin-like proteins), its C-terminal tail and seven lysine residues through which polyubiquitin chains are linked.
- Proteasome
-
The 26S proteasome is a protease complex that degrades polyubiquitylated proteins. It is composed of two subcomplexes: a barrel-shaped 20S core particle containing the protease active sites and two 19S regulatory particles that cap the barrel and control access of substrates to the core.
- Nuclear factor-κB
-
(NF-κB). A transcription factor with a key role in regulating the immune response. NF-κB is involved in cellular responses to stimuli, including stress, cytokines, free radicals, ultraviolet irradiation and bacterial or viral antigens. Misregulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection and improper immune development.
- HECT domain
-
A domain of ∼350 amino acids found at the C terminus of HECT E3s. The HECT domain contains a catalytic cysteine residue that accepts ubiquitin from an E2 to form a thioester intermediate before transferring ubiquitin to a substrate lysine.
- U-box
-
A domain comprising ∼70 amino acids that possesses a tertiary structure resembling the RING finger domain. The major difference is that the Ubox lacks the characteristic zinc-chelating cysteine and histidine residues of the RING finger. Consequently, Ubox E3s use intramolecular interactions other than zinc chelation to maintain the RING finger motif.
- RING finger
-
A domain present in most E3s that is defined by the consensus sequence C-X2-C-X[9-39]-C-X[1-3]-H-X[2-3]-C-X2-C-X[4-48]-C-X2-C (where X means any amino acid). The RING domain coordinates two zinc ions.
- Cullin-RING ligases
-
(CRLs). CRLs are a large family of multi-component E3s consisting of a core cullin protein bound to a RING finger protein (Rbx1/2), and an interchangeable substrate-binding adaptor protein. There are seven cullins and ∼600 adaptors in the human genome. Modification of the cullin subunit by NEDD8 is required for activation of CRL E3 ligase activity.
- SCF
-
SCF complexes are cullin RING ligases (CRLs) that catalyse the ubiquitylation of proteins targeted to the proteasome for degradation. SCF core subunits include the structural protein cullin 1, the RING-finger protein RBX1/2 and the adaptor protein Skp1. This core complex binds to one of the approximately 100 F-box proteins that are responsible for recruiting substrates. F-box proteins are named for the conserved 50 amino acid F-box domain that binds to SKP1. All CRLs, including SCFs, require NEDD8 modification of the cullin subunit for ligase activity.
- Rapamycin
-
Rapamycin (sirolimus) is a macrocyclic antibiotic produced by a bacterium isolated from soil on Easter Island. Rapamycin binds the cytosolic protein FK-binding protein 12 (FKBP12). The rapamcyin–FKBP12 complex inhibits the mTOR (mammalian target of rapamycin) pathway by directly binding mTOR complex1 (mTORC1).
- Cysteine proteases
-
This class of protease uses a cysteine thiol group in its catalytic mechanism. Deprotonation of the cysteine sulphydryl by an adjacent residue (usually histidine) is followed by nucleophilic attack on the peptide carbonyl carbon. A thioester linking the new C terminus to the cysteine thiol is an intermediate of the reaction.
- Zinc metalloproteases
-
A class of protease for which the active sites include two histidine residues that coordinate a zinc ion. During catalysis, the Zn2+ promotes attack of the peptide carbonyl carbon by the oxygen atom of a water molecule at the active site. An active site base facilitates this reaction by extracting a proton from the attacking water molecule.
- Autophagy
-
Literally means 'self-eating'; a highly regulated catabolic process in which cellular proteins and organelles are sequestered in a characteristic double-membrane vesicle called an autophagosome and are then degraded following vesicular fusion with a lysosome.
- Endosome–lysosome pathway
-
Endosomes are membrane- bound vesicles that are involved in protein transport between the plasma membrane, Golgi and lysosomes. In the endocytic pathway, internalized molecules are delivered to early endosomes, where efficient sorting occurs. Some molecules, including recycling receptors, are shunted back to the plasma membrane to be reused. Others, including downregulated receptors, are transported to late endosomes and lysosomes for degradation.
- Lewy bodies
-
Lewy bodies are abnormal protein aggregates that develop inside nerve cells in Parkinson's disease and Alzheimer's disease and some other disorders. They are identified when histology is performed on the brain and appear as spherical masses that displace other cell components.
Rights and permissions
About this article
Cite this article
Bedford, L., Lowe, J., Dick, L. et al. Ubiquitin-like protein conjugation and the ubiquitin–proteasome system as drug targets. Nat Rev Drug Discov 10, 29–46 (2011). https://doi.org/10.1038/nrd3321
Published:
Issue date:
DOI: https://doi.org/10.1038/nrd3321
This article is cited by
-
Structure-guided engineering enables E3 ligase-free and versatile protein ubiquitination via UBE2E1
Nature Communications (2024)
-
Personalized Protein-Protein Interaction Networks Towards Unraveling the Molecular Mechanisms of Alzheimer’s Disease
Molecular Neurobiology (2024)
-
Mesenchymal stem cell-derived extracellular vesicles subvert Th17 cells by destabilizing RORγt through posttranslational modification
Experimental & Molecular Medicine (2023)
-
The APC/C E3 ligase subunit ANAPC11 mediates FOXO3 protein degradation to promote cell proliferation and lymph node metastasis in urothelial bladder cancer
Cell Death & Disease (2023)
-
Nature-inspired protein ligation and its applications
Nature Reviews Chemistry (2023)