Key Points
-
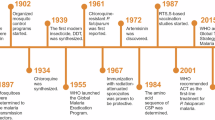
Antimalarial drug discovery has substantially contributed to the gains achieved against malaria over the past two decades. However, this progress and achieving the United Nations' Millennium Goals as they relate to malaria are both being threatened by emerging drug resistance, and major gaps persist for most target compound profiles.
-
Over the past decade, new, less costly and more sophisticated phenotypic screens have been developed, resulting in new drug candidates and antimalarial targets. Many of these molecules are now entering clinical development.
-
Four new molecules with unprecedented modes of action are currently in Phase II trials for blood-stage treatments: KAE609 (also known as cipargamin), OZ439 (also known as artefenomel), KAF156 (also known as GNF156) and DSM265. Each of these has the potential to shorten the current 3-day regimen of artemisinin combination therapies.
-
Significant contributions to the antimalarial drug discovery pipeline are being made by companies, academic institutions and non-profit organizations located in malaria-endemic countries. Moreover, Phase I studies are now being performed in populations that most need these antimalarials.
-
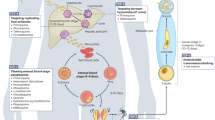
A new translational tool has been developed whereby antimalarial pharmacodynamics is studied in subclinically infected volunteers. The model is now routinely used for evaluating new drug candidates, resulting in significant time and cost savings for development.
-
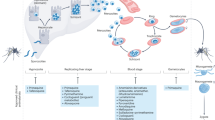
Clinical efforts are needed to develop treatments for vulnerable populations such as young children and expectant mothers, and for chemoprotection. Efforts are also needed for drugs that target the liver schizont stage of malaria parasites and for preventing transmission.
-
Progress has been made in early open-access drug discovery, with increased availability of proprietary compounds for screening assays.
Abstract
Despite substantial scientific progress over the past two decades, malaria remains a worldwide burden that causes hundreds of thousands of deaths every year. New, affordable and safe drugs are required to overcome increasing resistance against artemisinin-based treatments, treat vulnerable populations, interrupt the parasite life cycle by blocking transmission to the vectors, prevent infection and target malaria species that transiently remain dormant in the liver. In this Review, we discuss how the antimalarial drug discovery pipeline has changed over the past 10 years, grouped by the various target compound or product profiles, to assess progress and gaps, and to recommend priorities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Whitfield, J. Portrait of a serial killer: a roundup of the history and biology of the malaria parasite. Nature http://dx.doi.org/10.1038/news021001-6 (2002).
World Health Organization. World Malaria Report (WHO, 2014). A country-by-country overview of issues and gains made against malaria.
Sachs, J. & Malaney, P. The economic and social burden of malaria. Nature 415, 680–685 (2002).
Sicuri, E., Vieta, A., Lindner, L., Constenla, D. & Sauboin, C. The economic costs of malaria in children in three sub-Saharan countries: Ghana, Tanzania and Kenya. Malar. J. 12, 307 (2013).
Wells, T. N., Alonso, P. L. & Gutteridge, W. E. New medicines to improve control and contribute to the eradication of malaria. Nature Rev. Drug Discov. 8, 879–891 (2009).
Anthony, M. P., Burrows, J. N., Duparc, S., Moehrle, J. & Wells, T. N. C. The global pipeline of new medicines for the control and elimination of malaria. Malar. J. 11, 316 (2012).
Biamonte, M. A., Wanner, J. & Le Roch, K. G. Recent advances in malaria drug discovery. Bioorg. Med. Chem. Lett. 23, 2829–2843 (2013).
Alonso, P. L. et al. A research agenda to underpin malaria eradication. PLoS Med. 8, e1000406 (2011).
malERA Consultative Group on Drugs. A research agenda for malaria eradication: drugs. PLoS Med. 8, e1000402 (2011).
Burrows, J. N., Hooft van Huijsduijnen, R., Möhrle, J. J., Oeuvray, C. & Wells, T. N. C. Designing the next generation of medicines for malaria control and eradication. Malar. J. 12, 187 (2013). Defines the various target compound and product profiles for the different types of antimalarials and their rationale.
Engwerda, C. R., Minigo, G., Amante, F. H. & McCarthy, J. S. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. 28, 515–521 (2012). Describes a revolutionary translational tool to establish the efficacy and pharmacodynamics of candidate antimalarials in subclinically infected volunteers.
Bosman, A. & Mendis, K. N. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am. J. Trop. Med. Hyg. 77 (Suppl. 6), 193–197 (2007).
Noedl, H. et al. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359, 2619–2620 (2008).
Dondorp, A. M. et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361, 455–467 (2009).
Phyo, A. P. et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379, 1960–1966 (2012).
Ariey, F. et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505, 50–55 (2014). Describes the discovery of the genetic basis of resistance against artemisinins.
Miotto, O. et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nature Genet. 47, 226–234 (2015).
Straimer, J. et al. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 347, 428–431 (2014).
Jong-wook, L. Global health improvement and WHO: shaping the future. Lancet 362, 2083–2088 (2003).
Owens, S. Malaria and the millennium development goals. Arch. Dis. Child. 100 (Suppl. 1), 53–56 (2015).
Roll Back Malaria Partnership. Key facts, figures and strategies. Roll Back Malaria [online], (2008).
Lengeler, C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst. Rev. 2004, CD000363 (2004).
Cohen, J. M. et al. Malaria resurgence: a systematic review and assessment of its causes. Malar. J. 11, 122 (2012).
Roll Back Malaria Partnership. The global malaria action plan for a malaria-free world. Roll Back Malaria [online], (2008).
Toe, K. H. et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis. 20, 1691–1696 (2014).
Ashley, E. A. et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 371, 411–423 (2014). Provides a snapshot of the extent and spread of resistance against artemisinins.
Bethell, D. et al. Artesunate dose escalation for the treatment of uncomplicated malaria in a region of reported artemisinin resistance: a randomized clinical trial. PLoS ONE 6, e19283 (2011).
Gatton, M. L., Martin, L. B. & Cheng, Q. Evolution of resistance to sulfadoxine-pyrimethamine in Plasmodium falciparum. Antimicrob. Agents Chemother. 48, 2116–2123 (2004). Describes the mechanism and spread of resistance to the sulfadoxine–pyrimethamine combination.
Vinayak, S. et al. Origin and evolution of sulfadoxine resistant Plasmodium falciparum. PLoS Pathog. 6, e1000830 (2010).
Harrington, W. E., Mutabingwa, T. K., Kabyemela, E., Fried, M. & Duffy, P. E. Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance. Clin. Infect. Dis. 53, 224–230 (2011).
Steketee, R. W. & ter Kuile, F. Single low-dose primaquine to reduce malaria transmission. Lancet Infect. Dis. 14, 91–92 (2014).
Baird, J. K. Neglect of Plasmodium vivax malaria. Trends Parasitol. 23, 533–539 (2007). Highlights the burden and drug discovery efforts against P. vivax.
Bolchoz, L. J., Budinsky, R. A., McMillan, D. C. & Jollow, D. J. Primaquine-induced hemolytic anemia: formation and hemotoxicity of the arylhydroxylamine metabolite 6-methoxy-8-hydroxylaminoquinoline. J. Pharmacol. Exp. Ther. 297, 509–515 (2001). Describesprimaquine-induced complications.
Llanos-Cuentas, A. et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet 383, 1049–1058 (2014).
Price, R. N. & Nosten, F. Single-dose radical cure of Plasmodium vivax: a step closer. Lancet 383, 1020–1021 (2014).
Gesase, S. et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in Northern Tanzania and the emergence of dhps resistance mutation at codon 581. PLoS ONE 4, e4569 (2009).
Adam, I., Khamis, A. H. & Elbashir, M. I. Prevalence and risk factors for Plasmodium falciparum malaria in pregnant women of eastern Sudan. Malar. J. 4, 18 (2005).
Masaninga, F. et al. Review of the malaria epidemiology and trends in Zambia. Asian Pac. J. Trop. Biomed. 3, 89–94 (2013).
Biot, C. et al. The antimalarial ferroquine: from bench to clinic. Parasite 18, 207–214 (2011).
Supan, C. et al. Pharmacokinetics of ferroquine, a novel 4-aminoquinoline, in asymptomatic carriers of Plasmodium falciparum infections. Antimicrob. Agents Chemother. 56, 3165–3173 (2012).
Chavain, N. et al. Investigation of the redox behavior of ferroquine, a new antimalarial. Mol. Pharm. 5, 710–716 (2008).
Zhu, C. & Cook, S. P. A concise synthesis of (+)-artemisinin. J. Am. Chem. Soc. 134, 13577–13579 (2012).
Kopetzki, D., Levesque, F. & Seeberger, P. H. A continuous-flow process for the synthesis of artemisinin. Chemistry 19, 5450–5456 (2013).
Levesque, F. & Seeberger, P. H. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem. Int. Ed. Engl. 51, 1706–1709 (2012).
Valecha, N. et al. Arterolane maleate plus piperaquine phosphate for treatment of uncomplicated Plasmodium falciparum malaria: a comparative, multicenter, randomized clinical trial. Clin. Infect. Dis. 55, 663–671 (2012).
Noedl, H. The need for new antimalarial drugs less prone to resistance. Curr. Pharm. Design 19, 266–269 (2013).
Dondorp, A. M. et al. Artemisinin resistance: current status and scenarios for containment. Nature Rev. Microbiol. 8, 272–280 (2010).
Witkowski, B. et al. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob. Agents Chemother. 57, 914–923 (2013).
White, T. E., Bushdid, P. B., Ritter, S., Laffan, S. B. & Clark, R. L. Artesunate-induced depletion of embryonic erythroblasts precedes embryolethality and teratogenicity in vivo. Birth Defects Res. B Dev. Reprod. Toxicol. 77, 413–429 (2006).
Nagelschmitz, J. et al. First assessment in humans of the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative artemisone. Antimicrob. Agents Chemother. 52, 3085–3091 (2008).
O'Neill, P. M. et al. Identification of a 1,2,4,5-tetraoxane antimalarial drug-development candidate (RKA 182) with superior properties to the semisynthetic artemisinins. Angew. Chem. Int. Ed. Engl. 49, 5693–5697 (2010).
Mzayek, F. et al. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin. Trials 2, e6 (2007).
Thelingwani, R., Bonn, B., Chibale, K. & Masimirembwa, C. Physicochemical and drug metabolism characterization of a series of 4-aminoquinoline-3-hydroxypyridin-4-one hybrid molecules with antimalarial activity. Expert Opin. Drug Metab. Toxicol. 10, 1313–1324 (2014).
van Noord, C., Eijgelsheim, M. & Stricker, B. H. Drug- and non-drug-associated QT interval prolongation. Br. J. Clin. Pharmacol. 70, 16–23 (2010).
Zani, B., Gathu, M., Donegan, S., Olliaro, P. L. & Sinclair, D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst. Rev. 1, CD010927 (2014).
Wells, T. N. C. Is the tide turning for new malaria medicines? Science 329, 1153–1154 (2010).
Coteron, J. M. et al. Structure-guided lead optimization of triazolopyrimidine-ring substituents identifies potent Plasmodium falciparum dihydroorotate dehydrogenase inhibitors with clinical candidate potential. J. Med. Chem. 54, 5540–5561 (2011).
Deng, X. et al. Structural plasticity of malaria dihydroorotate dehydrogenase allows selective binding of diverse chemical scaffolds. J. Biol. Chem. 284, 26999–27009 (2009).
McCarthy, J. S. et al. A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS ONE 6, e21914 (2011).
Meister, S. et al. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334, 1372–1377 (2011).
Gamo, F. J. et al. Thousands of chemical starting points for antimalarial lead identification. Nature 465, 305–310 (2010). Describes how access to nearly 2 million compounds from GlaxoSmithKline resulted in the discovery of thousands of new inhibitors against Plasmodium spp.
Guiguemde, W. A. et al. Chemical genetics of Plasmodium falciparum. Nature 465, 311–315 (2010).
Spangenberg, T. et al. The open access malaria box: a drug discovery catalyst for neglected diseases. LoS ONE 8, e62906 (2013).
Avery, V. M. et al. Screening and hit evaluation of a chemical library against blood-stage Plasmodium falciparum. Malar. J. 13, 190 (2014).
Rottmann, M. et al. Spiroindolones, a potent compound class for the treatment of malaria. Science 329, 1175–1180 (2010).
Yeung, B. K. et al. Spirotetrahydro β-carbolines (spiroindolones): a new class of potent and orally efficacious compounds for the treatment of malaria. J. Med. Chem. 53, 5155–5164 (2010).
Spillman, N. J. et al. Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 13, 227–237 (2013).
Krishna, S. et al. Expression and functional characterization of a Plasmodium falciparum Ca2+-ATPase (PfATP4) belonging to a subclass unique to apicomplexan organisms. J. Biol. Chem. 276, 10782–10787 (2001).
Leong, F. J. et al. A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel antimalarial spiroindolone KAE609 (cipargamin), to assess the safety, tolerability and pharmacokinetics in healthy adult volunteers. Antimicrob. Agents Chemother. 58, 6209–6214 (2014). Describes the Phase I study of KAE609.
White, N. J. et al. Spiroindolone KAE609 for falciparum and vivax malaria. N. Engl. J. Med. 371, 403–410 (2014).
Cully, M. Trial watch: next-generation antimalarial from phenotypic screen shows clinical promise. Nature Rev. Drug Discov. 13, 717 (2014).
Flannery, E. L., Chatterjee, A. K. & Winzeler, E. A. Antimalarial drug discovery — approaches and progress towards new medicines. Nature Rev. Microbiol. 11, 849–862 (2013).
Das, S. et al. in The Annual Symposium of the Institute for Molecular Medicine & Infectious Disease Abstract B11, 46 (Drexel Univ. College of Medicine, 2014).
Vaidya, A. B. et al. Pyrazoleamide compounds are potent antimalarials that target Na+ homeostasis in intraerythrocytic Plasmodium falciparum. Nature Commun. 5, 5521 (2014).
Jimenez-Diaz, M. B. et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl Acad. Sci. USA 111, E5455–E5462 (2014).
Lehane, A. M., Ridgway, M. C., Baker, E. & Kirk, K. Diverse chemotypes disrupt ion homeostasis in the malaria parasite. Mol. Microbiol. 94, 327–339 (2014).
Younis, Y. et al. 3,5-diaryl-2-aminopyridines as a novel class of orally active antimalarials demonstrating single dose cure in mice and clinical candidate potential. J. Med. Chem. 55, 3479–3487 (2012).
Ghidelli-Disse, S. et al. Identification of Plasmodium PI4 kinase as target of MMV390048 by chemoproteomics. Malar. J. 13, S21 (2014).
McNamara, C. W. et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504, 248–253 (2013). Describes the discovery of PfATP4 as an antimalarial drug target.
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nature Rev. Drug Discov. 5, 993–996 (2006).
Gileadi, O. et al. The scientific impact of the Structural Genomics Consortium: a protein family and ligand-centered approach to medically-relevant human proteins. J. Struct. Funct. Genom. 8, 107–119 (2007).
Mullard, A. European lead factory opens for business. Nature Rev. Drug Discov. 12, 173–175 (2013).
Carroll, J. Will combinatorial chemistry keep its promise? Biotechnol. Healthc. 2, 26–32 (2005).
Geysen, H. M., Schoenen, F., Wagner, D. & Wagner, R. Combinatorial compound libraries for drug discovery: an ongoing challenge. Nature Rev. Drug Discov. 2, 222–230 (2003).
Wells, T. N. Natural products as starting points for future anti-malarial therapies: going back to our roots? Malar. J. 10 (Suppl. 1), 3 (2011).
Wells, T. N. Discovering and developing new medicines for malaria control and elimination. Infect. Disord. Drug Targets 13, 292–302 (2013).
Holmes, D. The GHIT fund shows its cards. Nature Rev. Drug Discov. 12, 894 (2013).
Slingsby, B. T. & Kurokawa, K. The Global Health Innovative Technology (GHIT) Fund: financing medical innovations for neglected populations. Lancet Glob. Health 1, e184–e185 (2013).
Zhang, Y. K. et al. Synthesis and structure–activity relationships of novel benzoxaboroles as a new class of antimalarial agents. Bioorg. Med. Chem. Lett. 21, 644–651 (2011).
Duffy, S. & Avery, V. M. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar. J. 12, 408 (2013).
Dembele, L. et al. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nature Med. 20, 307–312 (2014).
March, S. et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14, 104–115 (2013).
Hayden, F. G. Experimental human influenza: observations from studies of influenza antivirals. Antivir. Ther. 17, 133–141 (2012).
Atmar, R. L. et al. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14, 1553–1557 (2008).
Cordey, S. et al. Rhinovirus genome evolution during experimental human infection. PLoS ONE 5, e10588 (2010).
Spring, M., Polhemus, M. & Ockenhouse, C. Controlled human malaria infection. J. Infect. Dis. 209 (Suppl. 2), 40–45 (2014).
Epstein, J. E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334, 475–480 (2011).
Bousema, T. et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS ONE 7, e42821 (2012).
Price, R. N., Baird, J. K. & Hay, S. I. The Epidemiology of Plasmodium vivax: History, Hiatus and Hubris (eds. Rollinson, D. & Stothard, R.) (Elsevier, 2012).
Campbell, M. C. & Tishkoff, S. A. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu. Rev. Genom. Hum. Genet. 9, 403–433 (2008).
Ma, Q. & Lu, A. Y. Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacol. Rev. 63, 437–459 (2011).
Pang, T. Impact of pharmacogenomics on neglected diseases of the developing world. Am. J. Pharmacogenomics 3, 393–398 (2003).
Schuhmacher, A., Germann, P. G., Trill, H. & Gassmann, O. Models for open innovation in the pharmaceutical industry. Drug Discov. Today 18, 1133–1137 (2013).
Murphy, R. C. et al. Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med. Chem. Lett. 1, 331–335 (2010).
Bessoff, K. et al. Identification of Cryptosporidium parvum active chemical series by repurposing the open access malaria box. Antimicrob. Agents Chemother. 58, 2731–2739 (2014).
Boyom, F. F. et al. Repurposing the open access malaria box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob. Agents Chemother. 58, 5848–5854 (2014).
Aleman Resto, Y. & Fernandez Robledo, J. A. Identification of MMV malaria box inhibitors of Perkinsus marinus using an ATP-based bioluminescence assay. PLoS ONE 9, e111051 (2014).
Ingram-Sieber, K. et al. Orally active antischistosomal early leads identified from the open access malaria box. PLoS Negl. Trop. Dis. 8, e2610 (2014).
Doolan, D. L., Dobano, C. & Baird, J. K. Acquired immunity to malaria. Clin. Microbiol. Rev. 22, 13–36 (2009).
Rappuoli, R. & Aderem, A. A. 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature 473, 463–469 (2011).
RTS,S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 11, e1001685 (2014).
World Health Organization. Global malaria vaccine pipeline. WHO[online], (2014).
Seder, R. A. et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013).
McAllister, M. M. Successful vaccines for naturally occurring protozoal diseases of animals should guide human vaccine research. A review of protozoal vaccines and their designs. Parasitology 141, 624–640 (2014).
Petrovsky, N. & Aguilar, J. C. Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 82, 488–496 (2004).
Alving, C. R., Peachman, K. K., Rao, M. & Reed, S. G. Adjuvants for human vaccines. Curr. Opin. Immunol. 24, 310–315 (2012).
Kyes, S. A., Kraemer, S. M. & Smith, J. D. Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. Eukaryot. Cell 6, 1511–1520 (2007).
Kyes, S. et al. Plasmodium falciparum var gene expression is developmentally controlled at the level of RNA polymerase II-mediated transcription initiation. Mol. Microbiol. 63, 1237–1247 (2007).
Stockdale, C., Swiderski, M. R., Barry, J. D. & McCulloch, R. Antigenic variation in Trypanosoma brucei: joining the DOTs. PLoS Biol. 6, e185 (2008).
Ubben, D. & Poll, E. M. MMV in partnership: the Eurartesim® experience. Malar. J. 12, 211 (2013).
Dondorp, A. et al. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366, 717–725 (2005).
Dondorp, A. M. et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376, 1647–1657 (2010).
Ritchie, E. C., Block, J. & Nevin, R. L. Psychiatric side effects of mefloquine: applications to forensic psychiatry. J. Am. Acad. Psychiatry Law 41, 224–235 (2013).
Tansley, R. et al. A randomized, double-blind, placebo-controlled study to investigate the safety, tolerability, and pharmacokinetics of single enantiomer (+)-mefloquine compared with racemic mefloquine in healthy persons. Am. J. Trop. Med. Hyg. 83, 1195–1201 (2010).
Peters, P. J., Thigpen, M. C., Parise, M. E. & Newman, R. D. Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 30, 481–501 (2007).
Meremikwu, M. M., Donegan, S., Sinclair, D., Esu, E. & Oringanje, C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst. Rev. 2, CD003756 (2012).
Bueno, J. M. et al. Exploration of 4(1H)-pyridones as a novel family of potent antimalarial inhibitors of the plasmodial cytochrome bc1. Future Med. Chem. 4, 2311–2323 (2012).
Capper, M. J. et al. Antimalarial 4(1H)-pyridones bind to the Qi site of cytochrome bc1 . Proc. Natl Acad. Sci. USA 112, 755–760 (2015).
Powles, M. A. et al. MK-4815, a potential new oral agent for treatment of malaria. Antimicrob. Agents Chemother. 56, 2414–2419 (2012).
Macareo, L. et al. Triangular test design to evaluate tinidazole in the prevention of Plasmodium vivax relapse. Malar. J. 12, 173 (2013).
Bousejra-El Garah, F., Claparols, C., Benoit-Vical, F., Meunier, B. & Robert, A. The antimalarial trioxaquine DU1301 alkylates heme in malaria-infected mice. Antimicrob. Agents Chemother. 52, 2966–2969 (2008).
Dwivedi, A. et al. Role of type-II pathway in apoptotic cell death induction by photosensitized CDRI-97/78 under ambient exposure of UV-B. Toxicol. Lett. 222, 122–131 (2013).
Kushwaha, H. N. et al. Intersex effect of lamotrigine on the pharmacokinetic parameters of CDRI-97/78, a novel trioxane antimalarial compound, in rats. Arzneimittelforschung 62, 274–279 (2012).
Kushwaha, H. N. et al. Effect of carbamazepine on the pharmacokinetic parameters of CDRI-97/78 following coadministration to rats. Drug Res. (Stuttg.) 63, 282–288 (2013).
Shafiq, N. et al. Single ascending dose safety and pharmacokinetics of CDRI-97/78: first-in-human study of a novel antimalarial drug. Malar. Res. Treat. 2014, 372521 (2014).
Mombo-Ngoma, G. et al. Phase I randomized dose-ascending placebo-controlled trials of ferroquine — a candidate anti-malarial drug — in adults with asymptomatic Plasmodium falciparum infection. Malar. J. 10, 53 (2011).
Lanaspa, M. et al. Inadequate efficacy of a new formulation of fosmidomycin–clindamycin combination in Mozambican children less than three years old with uncomplicated Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 56, 2923–2928 (2012).
Lakshminarayana, S. B. et al. Pharmacokinetics–pharmacodynamics analysis of spiroindolone analogs and KAE609 in a murine malaria model. Antimicrob. Agents Chemother. 59, 1200–1210 (2014).
Kuhen, K. L. et al. KAF156 is an antimalarial clinical candidate with potential for use in prophylaxis, treatment and prevention of disease transmission. Antimicrob. Agents Chemother. 58, 5060–5067 (2014).
Guttmann, P. & Ehrlich, P. Über die wirkung des methylenblau bei malaria. Berliner Klinische Wochenschrift 28, 953–956 (in German) (1891).
Coulibaly, B. et al. Efficacy and safety of triple combination therapy with artesunate-amodiaquine-methylene blue for falciparum malaria in children: a randomized controlled trial in Burkina Faso. J. Infect. Dis. 211, 689–687 (2014).
Ciana, C. L. et al. Novel in vivo active anti-malarials based on a hydroxy-ethyl-amine scaffold. Bioorg. Med. Chem. Lett. 23, 658–662 (2013).
Bruderer, S. et al. First-in-humans study of the safety, tolerability, and pharmacokinetics of ACT-451840, a new chemical entity with antimalarial activity. Antimicrob. Agents Chemother. 59, 935–942 (2015).
Nanayakkara, N. P. et al. Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate. Antimicrob. Agents Chemother. 52, 2130–2137 (2008).
Marcsisin, S. R. et al. Tafenoquine and NPC-1161B require CYP2D metabolism for anti-malarial activity: implications for the 8-aminoquinoline class of anti-malarial compounds. Malar. J. 13, 2 (2014).
Marti, F. et al. Second generation analogues of RKA182: synthetic tetraoxanes with outstanding in vitro and in vivo antimalarial activities. MedChemComm 2, 661–665 (2011).
Copple, I. M. et al. Examination of the cytotoxic and embryotoxic potential and underlying mechanisms of next-generation synthetic trioxolane and tetraoxane antimalarials. Mol. Med. 18, 1045–1055 (2012).
Yuthavong, Y. et al. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc. Natl Acad. Sci. USA 109, 16823–16828 (2012).
Jomaa, H. et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285, 1573–1576 (1999).
Deng, X. et al. Fluorine modulates species selectivity in the triazolopyrimidine class of Plasmodium falciparum dihydroorotate dehydrogenase inhibitors. J. Med. Chem. 57, 5381–5394 (2014).
da Cruz, F. P. et al. Drug screen targeted at Plasmodium liver stages identifies a potent multistage antimalarial drug. J. Infect. Dis. 205, 1278–1286 (2012).
Nam, T. G. et al. A chemical genomic analysis of decoquinate, a Plasmodium falciparum cytochrome b inhibitor. ACS Chem. Biol. 6, 1214–1222 (2011).
Wang, J., Kaiser, M. & Copp, B. R. Investigation of indolglyoxamide and indolacetamide analogues of polyamines as antimalarial and antitrypanosomal agents. Mar. Drugs 12, 3138–3160 (2014).
Salas-Sarduy, E. et al. Antiparasitic effect of a fraction enriched in tight-binding protease inhibitors isolated from the Caribbean coral Plexaura homomalla. Exp. Parasitol. 135, 611–622 (2013).
Hansen, F. K. et al. Discovery of HDAC inhibitors with potent activity against multiple malaria parasite life cycle stages. Eur. J. Med. Chem. 82, 204–213 (2014).
Trenholme, K. et al. Lysine acetylation in sexual stage malaria parasites is a target for antimalarial small molecules. Antimicrob. Agents Chemother. 58, 3666–3678 (2014).
Sumanadasa, S. D. et al. Antimalarial activity of the anticancer histone deacetylase inhibitor SB939. Antimicrob. Agents Chemother. 56, 3849–3856 (2012).
Jaudzems, K. et al. Plasmepsin inhibitory activity and structure-guided optimization of a potent hydroxyethylamine-based antimalarial hit. ACS Med. Chem. Lett. 5, 373–377 (2014).
Meyers, M. J. et al. Evaluation of aminohydantoins as a novel class of antimalarial agents. ACS Med. Chem. Lett. 5, 89–93 (2014).
McCarthy, J. S. et al. A Phase I/Ib study to investigate the safety, tolerability and pharmacokinetic profile of DSM265 in healthy subjects and then its antimalarial activity in induced blood stage Plasmodium falciparum infection. ASTMH Ann. Meeting Abstr. 675, 204–205 (2014).
Acknowledgements
The authors thank the MMV team for helpful discussion, as well as our partners in the many projects and the continued support of our External Scientific Advisory Committees. We thank the reviewers of the original manuscript for many insightful suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All the authors are salaried (T.N.C.W.) or financially supported (R.H.v.H. and W.V.V.) by the Medicines for Malaria Venture, which has a stake in developing many of the drugs cited in this Review.
Related links
FURTHER INFORMATION
Glossary
- Chemotypes
-
Families of molecules with similar chemical structures that share biological or therapeutic effects. Many distinct chemotypes may have the same biological activity.
- Chemoprotection
-
In the context of malaria, chemoprotection refers to medicines that prevent healthy individuals from contracting malaria. Such drugs are used by travellers but are also considered for mass eradication campaigns.
- Target candidate profiles
-
A specific set of quantitative criteria that a molecule must be predicted of achieving before it is considered for development. These criteria typically involve biological activity, pharmacokinetics and, in malaria, cost of goods. The target product profile is the definition of the specifications for the final medicine, which, for malaria, may consist of several active molecules.
- Herd immunity
-
Immune protection that occurs when an entire population, including those without immunity themselves, is protected from an infectious disease owing to the high rate of immunity within that population.
- Stringent regulatory authorities
-
Regulatory agencies from the European Union (for example, the European Medicines Agency), the USA (the Food and Drug Administration) and Japan (the Ministry of Health, Labour, and Welfare), all of which are members of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), and other agencies that adhere to the same guidelines, such as in Switzerland, Australia and Canada.
- Prequalified
-
The WHO (World Health Organization) prequalification process is a formal assessment of diagnostics, medicines and vaccines, including review of the clinical dossier and the manufacturing and stability data, and site visits to manufacturers. This is a centralized procedure to approve products for purchase by specialized agencies of the United Nations.
- Blood stages
-
Early steps in the asexual, intra-erythrocytic replication cycle include the attachment of the Plasmodium spp. merozoites to blood cells, reorientation, irreversible attachment, junction formation, parasitophorous vacuole formation and invasion. The resulting immature trophozoite ring stage progresses into the mature tro-phozoite and schizont stages, which ruptures the blood cell to release new merozoites.
- Standard membrane feeding assays
-
Assays that measure the transmission of Plasmodium parasites back to mosquitoes; the insects' uptake of blood gametocytes and their successful mating is measured by counting (post-zygotic) midgut oocysts (the only route to re-infection). These assays involve mosquitoes feeding on human blood or serum through a membrane.
- Hypnozoites
-
Latent liver stages of Plasmodium vivax and Plasmodium ovale, species that are predominant in South and Southeast Asia and South America but only in Ethiopia and Sudan in Africa. Hypnozoites can remain dormant for weeks or even years, and carriers remain symptom-free until a relapse occurs.
- Human experimentally induced infection model
-
A model for malaria in which human volunteers are infected with Plasmodium parasites either by injection with infected erythrocytes (or sporozoites) or from the bite of an infected mosquito. Valuable pharmacokinetic and pharmacodynamic information of candidate drugs can thus be obtained at safe, well-monitored parasitaemia levels that are many orders of magnitude below those that induce symptoms.
- Heteroatoms
-
In organic chemistry, this term refers to all atoms that are not C or H. In the context of medicinal chemistry, novel heteroatoms refer to atoms not usually found in drugs (which are C, H, O, N, S and halogens).
- Gametocytes
-
Asexually replicating Plasmodium parasites in erythrocytes occasionally differentiate into gametocytes. These forms of the parasite are the only forms that can be taken up by mosquitoes, mate and propagate disease.
Rights and permissions
About this article
Cite this article
Wells, T., van Huijsduijnen, R. & Van Voorhis, W. Malaria medicines: a glass half full?. Nat Rev Drug Discov 14, 424–442 (2015). https://doi.org/10.1038/nrd4573
Published:
Issue date:
DOI: https://doi.org/10.1038/nrd4573
This article is cited by
-
Synthesis, docking and biological evaluation of some novel [1,2,4] triazolo [5,1-b] quinazoline Schiff base derivatives
Journal of the Iranian Chemical Society (2024)
-
Identifying inhibitors of β-haematin formation with activity against chloroquine-resistant Plasmodium falciparum malaria parasites via virtual screening approaches
Scientific Reports (2023)
-
Expert perspectives on the introduction of Triple Artemisinin-based Combination Therapies (TACTs) in Southeast Asia: a Delphi study
BMC Public Health (2022)
-
In vivo antiplasmodial activities and acute toxicity assessment of two plant cocktail extracts commonly used among Southwestern Nigerians
Journal of Parasitic Diseases (2022)
-
Antimicrobial therapeutics isolated from algal source: retrospect and prospect
Biologia (2022)