Abstract
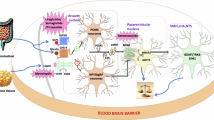
Obesity is a disorder characterized by an excess accumulation of body fat resulting from a mismatch between energy intake and expenditure. Incidence of obesity has increased dramatically in the past few years, almost certainly fuelled by a shift in dietary habits owing to the widespread availability of low-cost, hypercaloric foods. However, clear differences exist in obesity susceptibility among individuals exposed to the same obesogenic environment, implicating genetic risk factors. Numerous genes have been shown to be involved in the development of monofactorial forms of obesity. In genome-wide association studies, a large number of common variants have been associated with adiposity levels, each accounting for only a small proportion of the predicted heritability. Although the small effect sizes of obesity variants identified in genome-wide association studies currently preclude their utility in clinical settings, screening for a number of monogenic obesity variants is now possible. Such regular screening will provide more informed prognoses and help in the identification of at-risk individuals who could benefit from early intervention, in evaluation of the outcomes of current obesity treatments, and in personalization of the clinical management of obesity. This Review summarizes current advances in obesity genetics and discusses the future of research in this field and the potential relevance to personalized obesity therapy.
Key Points
-
Individual susceptibility to obesity is strongly influenced by genetic factors
-
Rare monogenic obesity variants of large effect size and common variants of small effect sizes collectively account for only a small proportion of the heritability of adiposity
-
Several copy number variants, both common and rare, have been shown to contribute to individual variation in BMI and increased risk of obesity
-
Genetic variants associated with BMI and obesity identified to date have increased our understanding of the underlying mechanisms by which obesity develops and is maintained
-
Screening for monofactorial obesity variants can provide informed prognoses for patients and opportunities for early intervention and treatment of additional pathologies frequently associated with these forms of obesity
-
Examination of clinical outcomes of interventions such as bariatric surgery in individuals with genetic susceptibility to obesity could permit personalization of obesity therapy in the future
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
05 November 2013
In the original published version of this article, the chromosomal positions of the loci in Figure 1b were incorrectly ordered. This error has now been corrected for the HTML and PDF versions of the article.
References
Haslam, D. W. & James, W. P. Obesity. Lancet 366, 1197–1209 (2005).
World Health Organization. Obesity and overweight [online], (2006).
World Health Organization: Obesity and overweight [online] (2012).
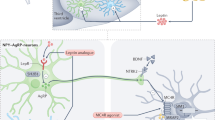
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Friedman, J. M. A war on obesity, not the obese. Science 299, 856–858 (2003).
Turula, M., Kaprio, J., Rissanen, A. & Koskenvuo, M. Body weight in the Finnish Twin Cohort. Diabetes Res. Clin. Pract. 10 (Suppl. 1), S33–S36 (1990).
Stunkard, A. J., Foch, T. T. & Hrubec, Z. A twin study of human obesity. JAMA 256, 51–54 (1986).
Stunkard, A. J., Harris, J. R., Pedersen, N. L. & McClearn, G. E. The body-mass index of twins who have been reared apart. N. Engl. J. Med. 322, 1483–1487 (1990).
Stunkard, A. J. et al. An adoption study of human obesity. N. Engl. J. Med. 314, 193–198 (1986).
Maes, H. H., Neale, M. C. & Eaves, L. J. Genetic and environmental factors in relative body weight and human adiposity. Behav. Genet. 27, 325–351 (1997).
Wardle, J., Carnell, S., Haworth, C. M. & Plomin, R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am. J. Clin. Nutr. 87, 398–404 (2008).
Silventoinen, K. et al. Modification effects of physical activity and protein intake on heritability of body size and composition. Am. J. Clin. Nutr. 90, 1096–1103 (2009).
Blakemore, A. I. & Froguel, P. Investigation of Mendelian forms of obesity holds out the prospect of personalized medicine. Ann. N. Y. Acad. Sci. 1214, 180–189 (2010).
O'Rahilly, S. Human genetics illuminates the paths to metabolic disease. Nature 462, 307–314 (2009).
Blakemore, A. I. & Froguel, P. Is obesity our genetic legacy? J. Clin. Endocrinol. Metab. 93, S51–S56 (2008).
Walley, A. J., Asher, J. E. & Froguel, P. The genetic contribution to non-syndromic human obesity. Nature Rev. Genet. 10, 431–442 (2009).
Ingalls, A. M., Dickie, M. M. & Snell, G. D. Obese, a new mutation in the house mouse. J. Hered. 41, 317–318 (1950).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
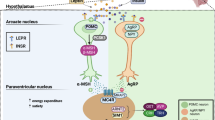
Wang, J., Liu, R., Hawkins, M., Barzilai, N. & Rossetti, L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393, 684–688 (1998).
Bado, A. et al. The stomach is a source of leptin. Nature 394, 790–793 (1998).
Hoggard, N. et al. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem. Biophys. Res. Commun. 232, 383–387 (1997).
Hoggard, N. et al. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc. Natl. Acad. Sci. USA 94, 11073–11078 (1997).
Tartaglia, L. A. et al. Identification and expression cloning of a leptin receptor, OB.-R. Cell 83, 1263–1271 (1995).
Chen, H. et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84, 491–495 (1996).
Lee, G. H. et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635 (1996).
Hummel, K. P., Dickie, M. M. & Coleman, D. L. Diabetes, a new mutation in the mouse. Science 153, 1127–1128 (1966).
Hummel, K. P., Coleman, D. L. & Lane, P. W. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains. Biochem. Genet. 7, 1–13 (1972).
Saeed, S., Butt, T. A., Anwer, M., Arslan, M. & Froguel, P. High prevalence of leptin and melanocortin-4 receptor gene mutations in children with severe obesity from Pakistani consanguineous families. Mol. Genet. Metab. 106, 121–126 (2012).
Ozata, M., Ozdemir, I. C. & Licinio, J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 84, 3686–3695 (1999).
Gibson, W. T. et al. Congenital leptin deficiency due to homozygosity for the Δ133G mutation: report of another case and evaluation of response to four years of leptin therapy. J. Clin. Endocrinol. Metab. 89, 4821–4826 (2004).
Paz-Filho, G. J. et al. Leptin replacement improves cognitive development. PLoS ONE 3, e3098 (2008).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903–908 (1997).
Farooqi, I. S. et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 (2002).
Strobel, A., Issad, T., Camoin, L., Ozata, M. & Strosberg, A. D. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 18, 213–215 (1998).
Farooqi, I. S. & O'Rahilly, S. Monogenic obesity in humans. Annu. Rev. Med. 56, 443–458 (2005).
Farooqi, I. S. & O'Rahilly, S. Leptin: a pivotal regulator of human energy homeostasis. Am. J. Clin. Nutr. 89, 980S–984S (2009).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999).
Farooqi, I. S. et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N. Engl. J. Med. 356, 237–247 (2007).
Clement, K. et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392, 398–401 (1998).
Mazen, I., El-Gammal, M., Abdel-Hamid, M., Farooqi, I. S. & Amr, K. Homozygosity for a novel missense mutation in the leptin receptor gene (P316T) in two Egyptian cousins with severe early onset obesity. Mol. Genet. Metab. 102, 461–4 (2011).
Pritchard, L. E., Turnbull, A. V. & White, A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J. Endocrinol. 172, 411–421 (2002).
Krude, H. & Gruters, A. Implications of proopiomelanocortin (POMC) mutations in humans: the POMC deficiency syndrome. Trends Endocrinol. Metab. 11, 15–22 (2000).
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19, 155–157 (1998).
Challis, B. G. et al. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum. Mol. Genet. 11, 1997–2004 (2002).
Creemers, J. W. et al. Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity. Diabetes 61, 383–390 (2012).
Jackson, R. S. et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J. Clin. Invest. 112, 1550–1560 (2003).
Jackson, R. S. et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 16, 303–306 (1997).
Farooqi, I. S. et al. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J. Clin. Endocrinol. Metab. 92, 3369–3373 (2007).
Dubern, B. et al. Mutational analysis of melanocortin-4 receptor, agouti-related protein, and alpha-melanocyte-stimulating hormone genes in severely obese children. J. Pediatr. 139, 204–209 (2001).
Stutzmann, F. et al. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 57, 2511–2518 (2008).
Farooqi, I. S. et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Invest. 106, 271–279 (2000).
Lubrano-Berthelier, C. et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J. Clin. Endocrinol. Metab. 91, 1811–1818 (2006).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095 (2003).
Holder, J. L., Jr, Butte, N. F. & Zinn, A. R. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 9, 101–108 (2000).
Kublaoui, B. M., Holder, J. L. Jr, Tolson, K. P., Gemelli, T. & Zinn, A. R. SIM1 overexpression partially rescues agouti yellow and diet-induced obesity by normalizing food intake. Endocrinology 147, 4542–4549 (2006).
Holder, J. L. Jr. et al. Sim1 gene dosage modulates the homeostatic feeding response to increased dietary fat in mice. Am. J. Physiol. Endocrinol. Metab. 287, E105–E113 (2004).
Michaud, J. L., Rosenquist, T., May, N. R. & Fan, C. M. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 12, 3264–3275 (1998).
Michaud, J. L. et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 10, 1465–1473 (2001).
Faivre, L. et al. Deletion of the SIM1 gene (6q16.2) in a patient with a Prader–Willi-like phenotype. J. Med. Genet. 39, 594–596 (2002).
Rankinen, T. et al. The human obesity gene map: the 2005 update. Obesity 14, 529–644 (2006).
Saunders, C. L. et al. Meta-analysis of genome-wide linkage studies in BMI and obesity. Obesity (Silver Spring) 15, 2263–2275 (2007).
Deng, H. W. et al. A genomewide linkage scan for quantitative-trait loci for obesity phenotypes. Am. J. Hum. Genet. 70, 1138–1151 (2002).
Liu, Y. J. et al. Tests of linkage and/or association of the LEPR gene polymorphisms with obesity phenotypes in Caucasian nuclear families. Physiol. Genomics 17, 101–106 (2004).
Stone, S. et al. A major predisposition locus for severe obesity, at 4p15-p14. Am. J. Hum. Genet. 70, 1459–1468 (2002).
Gorlova, O. Y. et al. Genetic linkage and imprinting effects on body mass index in children and young adults. Eur. J. Hum. Genet. 11, 425–432 (2003).
Norris, J. M. et al. Quantitative trait loci for abdominal fat and BMI in Hispanic-Americans and African-Americans: the IRAS Family study. Int. J. Obes. (Lond.) 29, 67–77 (2005).
Wilson, A. F., Elston, R. C., Tran, L. D. & Siervogel, R. M. Use of the robust sib-pair method to screen for single-locus, multiple-locus, and pleiotropic effects: application to traits related to hypertension. Am. J. Hum. Genet. 48, 862–872 (1991).
Moslehi, R., Goldstein, A. M., Beerman, M., Goldin, L. & Bergen, A. W. A genome-wide linkage scan for body mass index on Framingham Heart Study families. BMC Genet. 4, S97 (2003).
Cai, G. et al. Quantitative trait locus determining dietary macronutrient intakes is located on human chromosome 2p22. Am. J. Clin. Nutr. 80, 1410–1414 (2004).
Palmer, L. J. et al. A whole-genome scan for obstructive sleep apnea and obesity. Am. J. Hum. Genet. 72, 340–350 (2003).
Rotimi, C. N. et al. The quantitative trait locus on chromosome 2 for serum leptin levels is confirmed in African-Americans. Diabetes 48, 643–644 (1999).
Liu, Y. J. et al. A follow-up linkage study for quantitative trait loci contributing to obesity-related phenotypes. J. Clin. Endocrinol. Metab. 89, 875–882 (2004).
Fox, C. S. et al. Genome-wide linkage to chromosome 6 for waist circumference in the Framingham Heart Study. Diabetes 53, 1399–1402 (2004).
Wu, X. et al. A combined analysis of genomewide linkage scans for body mass index from the National Heart, Lung, and Blood Institute Family Blood Pressure Program. Am. J. Hum. Genet. 70, 1247–1256 (2002).
Luke, A. et al. Linkage for BMI at 3q27 region confirmed in an African-American population. Diabetes 52, 1284–1287 (2003).
Francke, S. et al. A genome-wide scan for coronary heart disease suggests in Indo-Mauritians a susceptibility locus on chromosome 16p13 and replicates linkage with the metabolic syndrome on 3q27. Hum. Mol. Genet. 10, 2751–2765 (2001).
Walder, K., Hanson, R. L., Kobes, S., Knowler, W. C. & Ravussin, E. An autosomal genomic scan for loci linked to plasma leptin concentration in Pima Indians. Int. J. Obes. Relat. Metab. Disord. 24, 559–565 (2000).
Kissebah, A. H. et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 97, 14478–14483 (2000).
Vionnet, N. et al. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am. J. Hum. Genet. 67, 1470–1480 (2000).
Atwood, L. D. et al. Genomewide linkage analysis of body mass index across 28 years of the Framingham Heart Study. Am. J. Hum. Genet. 71, 1044–1050 (2002).
Meyre, D. et al. A genome-wide scan for childhood obesity-associated traits in French families shows significant linkage on chromosome 6q22.31-q23.2. Diabetes 53, 803–811 (2004).
Feitosa, M. F. et al. Quantitative-trait loci influencing body-mass index reside on chromosomes 7 and 13: the National Heart, Lung, and Blood Institute Family Heart Study. Am. J. Hum. Genet. 70, 72–82 (2002).
Hsueh, W. C. et al. Genome-wide scan of obesity in the Old Order Amish. J. Clin. Endocrinol. Metab. 86, 1199–1205 (2001).
Perusse, L. et al. A genome-wide scan for abdominal fat assessed by computed tomography in the Quebec Family Study. Diabetes 50, 614–621 (2001).
Price, R. A. et al. A locus affecting obesity in human chromosome region 10p12. Diabetologia 44, 363–366 (2001).
Hager, J. et al. A genome-wide scan for human obesity genes reveals a major susceptibility locus on chromosome 10. Nat. Genet. 20, 304–308 (1998).
Hinney, A. et al. Independent confirmation of a major locus for obesity on chromosome 10. J. Clin. Endocrinol. Metab. 85, 2962–2965 (2000).
Hanson, R. L. et al. An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am. J. Hum. Genet. 63, 1130–1138 (1998).
Norman, R. A. et al. Genomewide search for genes influencing percent body fat in Pima Indians: suggestive linkage at chromosome 11q21-q22. Pima Diabetes Gene Group. Am. J. Hum. Genet. 60, 166–173 (1997).
Norman, R. A. et al. Autosomal genomic scan for loci linked to obesity and energy metabolism in Pima Indians. Am. J. Hum. Genet. 62, 659–668 (1998).
Dong, C. et al. Interacting genetic loci on chromosomes 20 and 10 influence extreme human obesity. Am. J. Hum. Genet. 72, 115–124 (2003).
Hunt, S. C. et al. Linkage of body mass index to chromosome 20 in Utah pedigrees. Hum. Genet. 109, 279–285 (2001).
Lee, J. H. et al. Genome scan for human obesity and linkage to markers in 20q13. Am. J. Hum. Genet. 64, 196–209 (1999).
Young, E. H. et al. The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29563 individuals. Int. J. Obes (Lond.) 31, 1437–1441 (2007).
Stutzmann, F. et al. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum. Mol. Genet. 16, 1837–1844 (2007).
Heid, I. M. et al. Association of the 103I MC4R allele with decreased body mass in 7937 participants of two population based surveys. J. Med. Genet. 42, e21 (2005).
Loos, R. J. et al. Melanocortin-4 receptor gene and physical activity in the Quebec Family Study. Int. J. Obes. (Lond.) 29, 420–428 (2005).
Geller, F. et al. Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. Am. J. Hum. Genet. 74, 572–581 (2004).
Ma, L., Tataranni, P. A., Bogardus, C. & Baier, L. J. Melanocortin 4 receptor gene variation is associated with severe obesity in Pima Indians. Diabetes 53, 2696–2699 (2004).
Hinney, A. et al. Melanocortin-4 receptor gene: case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J. Clin. Endocrinol. Metab. 88, 4258–4267 (2003).
Chagnon, Y. C. et al. Linkage and association studies between the melanocortin receptors 4 and 5 genes and obesity-related phenotypes in the Quebec Family Study. Mol. Med. 3, 663–673 (1997).
El-Gharbawy, A. H. et al. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. J. Clin. Endocrinol. Metab. 91, 3548–3552 (2006).
Ribases, M. et al. Association of BDNF with restricting anorexia nervosa and minimum body mass index: a family-based association study of eight European populations. Eur. J. Hum. Genet. 13, 428–434 (2005).
Gray, J. et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55, 3366–3371 (2006).
Shugart, Y. Y. et al. Two British women studies replicated the association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) and BMI. Eur. J. Hum. Genet. 17, 1050–1055 (2009).
Benzinou, M. et al. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat. Genet. 40, 943–945 (2008).
Fujisawa, T., Ikegami, H., Kawaguchi, Y. & Ogihara, T. Meta-analysis of the association of Trp64Arg polymorphism of β3-adrenergic receptor gene with body mass index. J. Clin. Endocrinol. Metab. 83, 2441–2444 (1998).
Kurokawa, N. et al. The ADRB3 Trp64Arg variant and BMI: a meta-analysis of 44 833 individuals. Int. J. Obes. (Lond.) 32, 1240–9 (2008).
Allison, D. B., Heo, M., Faith, M. S. & Pietrobelli, A. Meta-analysis of the association of the Trp64Arg polymorphism in the β3-adrenergic receptor with body mass index. Int. J. Obes. Relat. Metab. Disord. 22, 559–566 (1998).
Clement, K. et al. Genetic variation in the β3-adrenergic receptor and an increased capacity to gain weight in patients with morbid obesity. N. Engl. J. Med. 333, 352–354 (1995).
Kurokawa, N., Nakai, K., Kameo, S., Liu, Z. M. & Satoh, H. Association of BMI with the β3-adrenergic receptor gene polymorphism in Japanese: meta-analysis. Obes. Res. 9, 741–745 (2001).
Deeb, S. S. et al. A Pro12Ala substitution in PPARγ2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat. Genet. 20, 284–287 (1998).
Tonjes, A., Scholz, M., Loeffler, M. & Stumvoll, M. Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor γ with Pre-diabetic phenotypes: meta-analysis of 57 studies on nondiabetic individuals. Diabetes Care 29, 2489–2497 (2006).
Ristow, M., Muller-Wieland, D., Pfeiffer, A., Krone, W. & Kahn, C. R. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N. Engl. J. Med. 339, 953–959 (1998).
Loos, R. J. Recent progress in the genetics of common obesity. Br. J. Clin. Pharmacol. 68, 811–829 (2009).
Ichimura, A. et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483, 350–354 (2012).
Dina, C. et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 39, 724–726 (2007).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 (2007).
Scuteri, A. et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 3, e115 (2007).
Bradfield, J. P. et al. A genome-wide association meta-analysis identifies new childhood obesity loci. Nat. Genet. 44, 526–531 (2012).
Tan, J. T. et al. FTO variants are associated with obesity in the Chinese and Malay populations in Singapore. Diabetes 57, 2851–2857 (2008).
Dorajoo, R. et al. Replication of 13 obesity loci among Singaporean Chinese, Malay and Asian-Indian populations. Int. J. Obes (Lond.) 36, 159–163 (2012).
Paternoster, L. et al. Genome-wide population-based association study of extremely overweight young adults--the GOYA study. PLoS ONE 6, e24303 (2011).
Wang, H., Dong, S., Xu, H., Qian, J. & Yang, J. Genetic variants in FTO associated with metabolic syndrome: a meta- and gene-based analysis. Mol. Biol. Rep. 39, 5691–5698 (2012).
Heard-Costa, N. L. et al. NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS Genet. 5, e1000539 (2009).
Hinney, A. et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE 2, e1361 (2007).
Liu, J. Z. et al. Genome-wide association study of height and body mass index in Australian twin families. Twin Res. Hum. Genet. 13, 179–193 (2010).
Scherag, A. et al. Two new loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and German study groups. PLoS Genet. 6, e1000916 (2010).
Speliotes, E. K. et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 42, 937–948 (2010).
Meyre, D. et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat. Genet. 41, 157–159 (2009).
Loos, R. J. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 40, 768–775 (2008).
Thorleifsson, G. et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 41, 18–24 (2009).
Willer, C. J. et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41, 25–34 (2009).
Cotsapas, C. et al. Common body mass index-associated variants confer risk of extreme obesity. Hum. Mol. Genet. 18, 3502–3507 (2009).
Gerken, T. et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318, 1469–1472 (2007).
Melka, M. G. et al. FTO, obesity and the adolescent brain. Hum. Mol. Genet. (2012).
Ho, A. J. et al. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc. Natl Acad. Sci. USA 107, 8404–8409 (2010).
Chambers, J. C. et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat. Genet. 40, 716–8 (2008).
Wang, K. et al. A genome-wide association study on obesity and obesity-related traits. PLoS ONE 6, e18939 (2011).
Day, F. R. & Loos, R. J. Developments in obesity genetics in the era of genome-wide association studies. J. Nutrigenet. Nutrigenomics 4, 222–238 (2011).
Fall, T. & Ingelsson, E. Genome-wide association studies of obesity and metabolic syndrome. Mol. Cell. Endocrinol. (2012).
Redon, R. et al. Global variation in copy number in the human genome. Nature 444, 444–454 (2006).
Korbel, J. O. et al. Paired-end mapping reveals extensive structural variation in the human genome. Science 318, 420–426 (2007).
de Smith, A. J. et al. Array CGH analysis of copy number variation identifies 1284 new genes variant in healthy white males: implications for association studies of complex diseases. Hum. Mol. Genet. 16, 2783–2794 (2007).
Conrad, D. F. et al. Origins and functional impact of copy number variation in the human genome. Nature 464, 704–712 (2010).
Sha, B. Y. et al. Genome-wide association study suggested copy number variation may be associated with body mass index in the Chinese population. J. Hum. Genet. 54, 199–202 (2009).
Jarick, I. et al. Novel common copy number variation for early onset extreme obesity on chromosome 11q11 identified by a genome-wide analysis. Hum. Mol. Genet. 20, 840–852 (2011).
Walters, R. G. et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature 463, 671–675 (2010).
Bochukova, E. G. et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature 463, 666–670 (2010).
Wang, K. et al. Large copy-number variations are enriched in cases with moderate to extreme obesity. Diabetes 59, 2690–2694 (2010).
Yu, Y. et al. Age- and gender-dependent obesity in individuals with 16p11.2 deletion. J. Genet. Genomics 38, 403–409 (2011).
Weiss, L. A. et al. Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med. 358, 667–675 (2008).
McCarthy, S. E. et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat. Genet. 41, 1223–1227 (2009).
Jacquemont, S. et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature 478, 97–102 (2011).
Ren, D. et al. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J. Clin. Invest. 117, 397–406 (2007).
Doche, M. E. et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J. Clin. Invest. 122, 4732–4736 (2012).
Glessner, J. T. et al. A genome-wide study reveals copy number variants exclusive to childhood obesity cases. Am. J. Hum. Genet. 87, 661–666 (2010).
El-Sayed Moustafa, J. S. et al. Novel association approach for variable number tandem repeats (VNTRs) identifies DOCK5 as a susceptibility gene for severe obesity. Hum. Mol. Genet. 21, 3727–3738 (2012).
Wang, W. Y., Barratt, B. J., Clayton, D. G. & Todd, J. A. Genome-wide association studies: theoretical and practical concerns. Nat. Rev. Genet. 6, 109–118 (2005).
Loos, R. J. Genetic determinants of common obesity and their value in prediction. Best Pract. Res. Clin. Endocrinol. Metab. 26, 211–226 (2012).
Morandi, A. et al. Estimation of newborn risk for child or adolescent obesity: lessons from longitudinal birth cohorts. PLoS ONE 7, e49919 (2012).
Cassidy, S. B., Dykens, E. & Williams, C. A. Prader-Willi and Angelman syndromes: sister imprinted disorders. Am. J. Med. Genet. 97, 136–146 (2000).
Livshits, G., Malkin, I., Moayyeri, A., Spector, T. D. & Hammond, C. J. Association of FTO gene variants with body composition in UK twins. Ann. Hum. Genet. 76, 333–341 (2012).
Sarzynski, M. A. et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int. J. Obes. (Lond.) 35, 676–683 (2011).
Still, C. D. et al. High allelic burden of four obesity SNPs is associated with poorer weight loss outcomes following gastric bypass surgery. Obesity (Silver Spring) 19, 1676–1683 (2011).
Aslan, I. R. et al. Bariatric surgery in a patient with complete MC4R deficiency. Int. J. Obes. (Lond.) 35, 457–461 (2010).
Aslan, I. R. et al. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes. Surg. 21, 930–934 (2011).
Hatoum, I. J. et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J. Clin. Endocrinol. Metab. 97, E1023–E1031 (2012).
Valette, M. et al. Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLoS ONE 7, e48221 (2012).
Manolio, T. A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Bonnefond, A. et al. Molecular diagnosis of neonatal diabetes mellitus using next-generation sequencing of the whole exome. PLoS ONE 5, e13630 (2010).
Bonnefond, A. et al. Whole-Exome Sequencing and High Throughput Genotyping Identified KCNJ11 as the Thirteenth MODY Gene. PLoS ONE 7, e37423 (2012).
Hoischen, A. et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat. Genet. 42, 483–485 (2010).
Sanders, S. J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012).
Neale, B. M. et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245 (2012).
Golzio, C. et al. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature 485, 363–367 (2012).
Pruim, R. J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010).
Acknowledgements
The authors wish to thank S. Saeed and H. Al-Saud (Department of Genomics of Common Disease, Imperial College London, London, United Kingdom W12 0NN) for helpful suggestions and a critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
J. S. El-Sayed Moustafa and P. Froguel contributed equally to researching the data for the article, discussion of content, and to writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
El-Sayed Moustafa, J., Froguel, P. From obesity genetics to the future of personalized obesity therapy. Nat Rev Endocrinol 9, 402–413 (2013). https://doi.org/10.1038/nrendo.2013.57
Published:
Issue date:
DOI: https://doi.org/10.1038/nrendo.2013.57
This article is cited by
-
Salivary amylase gene (AMY1) copy number variation has only a minor correlation with body composition in Chinese adults
Genes & Genomics (2023)
-
Molecular profiling of melanocortin 4 receptor variants and agouti-related peptide interactions in morbid obese phenotype: a novel paradigm from molecular docking and dynamics simulations
Biologia (2022)
-
Transcriptome of visceral adipose tissue identifies an inflammation-related ceRNA network that regulates obesity
Molecular and Cellular Biochemistry (2022)
-
Genetic polymorphisms associated with obesity in the Arab world: a systematic review
International Journal of Obesity (2021)
-
Cardiovascular Diseases in Obesity: What is the Role of Magnesium?
Biological Trace Element Research (2021)