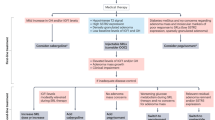
Abstract
In March 2013, the Acromegaly Consensus Group met to revise and update guidelines for the medical treatment of acromegaly. The meeting comprised experts skilled in the medical management of acromegaly. The group considered treatment goals covering biochemical, clinical and tumour volume outcomes, and the place in guidelines of somatostatin receptor ligands, growth hormone receptor antagonists and dopamine agonists, and alternative modalities for treatment including combination therapy and novel treatments. This document represents the conclusions of the workshop consensus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Melmed, S. et al. Consensus statement: medical management of acromegaly. Eur. J. Endocrinol. 153, 737–740 (2005).
Melmed, S. Acromegaly pathogenesis and treatment. J. Clin. Invest. 119, 3189–3202 (2009).
Giustina, A. et al. Current management practices for acromegaly: an international survey. Pituitary 14, 125–133 (2011).
Melmed, S. et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 16, 294–302 (2013).
Melmed, S. et al. Guidelines for acromegaly management: an update. J. Clin. Endocrinol. Metab. 94, 1509–1517 (2009).
Katznelson, L. et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly—2011 update: executive summary. Endocr. Pract. 17, 636–646 (2011).
Melmed, S. Medical progress: acromegaly. N. Engl. J. Med. 355, 2558–2573 (2006).
Funder, J. W. et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 93, 3266–3281 (2008).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008).
Holdaway, I. M., Rajasoorya, R. C. & Gamble, G. D. Factors influencing mortality in acromegaly. J. Clin. Endocrinol. Metab. 89, 667–674 (2004).
Holdaway, I. M., Bolland, M. J. & Gamble, G. D. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur. J. Endocrinol. 159, 89–95 (2008).
Clemmons, D. R. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin. Chem. 57, 555–559 (2011).
Giustina, A. et al. A consensus on criteria for cure of acromegaly. J. Clin. Endocrinol. Metab. 95, 3141–3148 (2010).
Carmichael, J. D., Bonert, V. S., Mirocha, J. M. & Melmed, S. The utility of oral glucose tolerance testing for diagnosis and assessment of treatment outcomes in 166 patients with acromegaly. J. Clin. Endocrinol. Metab. 94, 523–527 (2009).
Melmed, S. et al. A critical analysis of pituitary tumor shrinkage during primary medical therapy in acromegaly. J. Clin. Endocrinol. Metab. 90, 4405–4410 (2005).
Mazziotti, G. & Giustina, A. Effects of lanreotide SR and Autogel on tumor mass in patients with acromegaly: a systematic review. Pituitary 13, 60–67 (2010).
Giustina, A. et al. Meta-analysis on the effects of octreotide on tumor mass in acromegaly. PLoS ONE 7, e36411 (2012).
Colao, A., Auriemma, R. S., Galdiero, M., Lombardi, G. & Pivonello, R. Effects of initial therapy for five years with somatostatin analogs for acromegaly on growth hormone and insulin-like growth factor-I levels, tumor shrinkage, and cardiovascular disease: a prospective study. J. Clin. Endocrinol. Metab. 94, 3746–3756 (2009).
Colao, A. et al. Octreotide LAR vs. surgery in newly diagnosed patients with acromegaly: a randomized, open-label, multicentre study. Clin. Endocrinol. (Oxf.) 70, 757–768 (2009).
Ben-Shlomo, A., Sheppard, M. C., Stephens, J. M., Pulgar, S. & Melmed, S. Clinical, quality of life, and economic value of acromegaly disease control. Pituitary 14, 284–294 (2011).
Davi, M. V. et al. Sleep apnoea syndrome is highly prevalent in acromegaly and only partially reversible after biochemical control of the disease. Eur. J. Endocrinol. 159, 533–540 (2008).
Espinosa-de-los-Monteros, A. L., Gonzalez, B., Vargas, G., Sosa, E. & Mercado, M. Clinical and biochemical characteristics of acromegalic patients with different abnormalities in glucose metabolism. Pituitary 14, 231–235 (2011).
Bonadonna, S. et al. Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in postmenopausal women. J. Bone Miner. Res. 20, 1837–1844 (2005).
Salvatori, R. et al. Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naive patients with acromegaly. Pituitary 13, 115–122 (2010).
Yang, L. P. & Keating, G. M. Octreotide long-acting release (LAR): a review of its use in the management of acromegaly. Drugs 70, 1745–1769 (2010).
Toledano, Y. et al. Efficacy of long-term lanreotide treatment in patients with acromegaly. Pituitary 12, 285–293 (2009).
Schopohl, J. et al. Efficacy and acceptability of lanreotide Autogel®120 mg at different dose intervals in patients with acromegaly previously treated with octreotide LAR. Exp. Clin. Endocrinol. Diabetes 119, 156–162 (2011).
Murray, R. D. & Melmed, S. A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J. Clin. Endocrinol. Metab. 93, 2957–2968 (2008).
Petersenn, S. et al. Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J. Clin. Endocrinol. Metab. 95, 2781–2789 (2010).
Tuvia, S. et al. Oral octreotide absorption in human subjects: comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppression. J. Clin. Endocrinol. Metab. 97, 2362–2369 (2012).
Sandret, L., Maison, P. & Chanson, P. Place of cabergoline in acromegaly: a meta-analysis. J. Clin. Endocrinol. Metab. 96, 1327–1335 (2011).
Maione, L. et al. No evidence of a detrimental effect of cabergoline therapy on cardiac valves in patients with acromegaly. J. Clin. Endocrinol. Metab. 97, E1714–E1719 (2012).
van der Lely, A. J. et al. Long-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDY. J. Clin. Endocrinol. Metab. 97, 1589–1597 (2012).
Bonert, V. S. et al. Lipodystrophy in patients with acromegaly receiving pegvisomant. J. Clin. Endocrinol. Metab. 93, 3515–3518 (2008).
Garcia Basavilbaso, N. et al. Experience from the Argentine Pegvisomant Observational Study: preliminary data. Front. Horm. Res. 38, 42–49 (2010).
Sievers, C. et al. Change of symptoms and perceived health in acromegalic patients on pegvisomant therapy: a retrospective cohort study within the German Pegvisomant Observational Study (GPOS). Clin. Endocrinol. (Oxf.) 73, 89–94 (2010).
Trainer, P. J., Ezzat, S., D'Souza, G. A., Layton, G. & Strasburger, C. J. A randomized, controlled, multicentre trial comparing pegvisomant alone with combination therapy of pegvisomant and long-acting octreotide in patients with acromegaly. Clin. Endocrinol. (Oxf.) 71, 549–557 (2009).
Dekkers, O. M., Biermasz, N. R., Pereira, A. M., Romijn, J. A. & Vandenbroucke, J. P. Mortality in acromegaly: a metaanalysis. J. Clin. Endocrinol. Metab. 93, 61–67 (2008).
Burgers, A. M. et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J. Clin. Endocrinol. Metab. 96, 2912–2920 (2011).
Colao, A. et al. No greater incidence or worsening of cardiac valve regurgitation with somatostatin analog treatment of acromegaly. J. Clin. Endocrinol. Metab. 93, 2243–2248 (2008).
Gouya, H. et al. Rapidly reversible myocardial edema in patients with acromegaly: assessment with ultrafast T2 mapping in a single-breath-hold MRI sequence. AJR Am. J. Roentgenol. 190, 1576–1582 (2008).
Annamalai, A. K. et al. A comprehensive study of clinical, biochemical, radiological, vascular, cardiac, and sleep parameters in an unselected cohort of patients with acromegaly undergoing presurgical somatostatin receptor ligand therapy. J. Clin. Endocrinol. Metab. 98, 1040–1050 (2013).
Attal, P. & Chanson, P. Endocrine aspects of obstructive sleep apnea. J. Clin. Endocrinol. Metab. 95, 483–495 (2010).
Berg, C. et al. Influence of disease control with pegvisomant on sleep apnoea and tongue volume in patients with active acromegaly. Eur. J. Endocrinol. 161, 829–835 (2009).
Biermasz, N. R. et al. Automated image analysis of hand radiographs reveals widened joint spaces in patients with long-term control of acromegaly: relation to disease activity and symptoms. Eur. J. Endocrinol. 166, 407–413 (2012).
Claessen, K. M. et al. Increased clinical symptoms of acromegalic arthropathy in patients with long-term disease control: a prospective follow-up study. Pituitary 17, 44–52 (2014).
Mazziotti, G. et al. Prevalence of vertebral fractures in men with acromegaly. J. Clin. Endocrinol. Metab. 93, 4649–4655 (2008).
Battista, C. et al. Spinal volumetric trabecular bone mass in acromegalic patients: a longitudinal study. Clin. Endocrinol. (Oxf.) 70, 378–382 (2009).
Mazziotti, G. et al. Effects of somatostatin analogs on glucose homeostasis: a metaanalysis of acromegaly studies. J. Clin. Endocrinol. Metab. 94, 1500–1508 (2009).
Roset, M. et al. Cost of clinical management of acromegaly in Spain. Clin. Drug Investig. 32, 235–245 (2012).
Sesmilo, G. et al. Changes in acromegaly treatment over four decades in Spain: analysis of the Spanish Acromegaly Registry (REA). Pituitary 16, 115–121 (2013).
Bex, M. et al. AcroBel—the Belgian registry on acromegaly: a survey of the 'real-life' outcome in 418 acromegalic subjects. Eur. J. Endocrinol. 157, 399–409 (2007).
Bevan, J. S. Clinical review: the antitumoral effects of somatostatin analog therapy in acromegaly. J. Clin. Endocrinol. Metab. 90, 1856–1863 (2005).
van der Lely, A. J. et al. Control of tumor size and disease activity during cotreatment with octreotide and the growth hormone receptor antagonist pegvisomant in an acromegalic patient. J. Clin. Endocrinol. Metab. 86, 478–481 (2001).
Jimenez, C. et al. Follow-up of pituitary tumor volume in patients with acromegaly treated with pegvisomant in clinical trials. Eur. J. Endocrinol. 159, 517–523 (2008).
Buhk, J. H. et al. Tumor volume of growth hormone-secreting pituitary adenomas during treatment with pegvisomant: a prospective multicenter study. J. Clin. Endocrinol. Metab. 95, 552–558 (2010).
Marazuela, M. et al. Somatotroph tumor progression during pegvisomant therapy: a clinical and molecular study. J. Clin. Endocrinol. Metab. 96, E251–E259 (2011).
Carlsen, S. M. et al. Six-month preoperative octreotide treatment in unselected, de novo patients with acromegaly: effect on biochemistry, tumour volume, and postoperative cure. Clin. Endocrinol. (Oxf.) 74, 736–743 (2011).
Mao, Z. G. et al. Preoperative lanreotide treatment in acromegalic patients with macroadenomas increases short-term postoperative cure rates: a prospective, randomised trial. Eur. J. Endocrinol. 162, 661–666 (2010).
Shen, M. et al. Effect of presurgical long-acting octreotide treatment in acromegaly patients with invasive pituitary macroadenomas: a prospective randomized study. Endocr. J. 57, 1035–1044 (2010).
Pita-Gutierrez, F. et al. Place of preoperative treatment of acromegaly with somatostatin analog on surgical outcome: a systematic review and meta-analysis. PLoS ONE 8, e61523 (2013).
Trainer, P. J. ACROSTUDY: the first 5 years. Eur. J. Endocrinol. 161 (Suppl. 1), S19–S24 (2009).
Higham, C. E., Chung, T. T., Lawrance, J., Drake, W. M. & Trainer, P. J. Long-term experience of pegvisomant therapy as a treatment for acromegaly. Clin. Endocrinol. (Oxf.) 71, 86–91 (2009).
Neggers, S. J. & van der Lely, A. J. Combination treatment with somatostatin analogues and pegvisomant in acromegaly. Growth Horm. IGF Res. 21, 129–133 (2011).
Giustina, A. et al. High-dose intramuscular octreotide in patients with acromegaly inadequately controlled on conventional somatostatin analogue therapy: a randomised controlled trial. Eur. J. Endocrinol. 161, 331–338 (2009).
Mazziotti, G. et al. Effects of high-dose octreotide LAR on glucose metabolism in patients with acromegaly inadequately controlled by conventional somatostatin analog therapy. Eur. J. Endocrinol. 164, 341–347 (2011).
Fleseriu, M. Clinical efficacy and safety results for dose escalation of somatostatin receptor ligands in patients with acromegaly: a literature review. Pituitary 14, 184–193 (2011).
Auriemma, R. S. et al. Complete disappearance of a GH-secreting pituitary macroadenoma in a patient with acromegaly: effect of treatment with lanreotide Autogel and consequence of treatment withdrawal. Eur. J. Endocrinol. 162, 993–999 (2010).
Ozbek, M., Erdogan, M., Akbal, E. & Gonulalan, G. Disappearance of a GH secreting macroadenoma, during long-term somatostatin analogue administration. Exp. Clin. Endocrinol. Diabetes 117, 309–311 (2009).
Ramirez, C. et al. Discontinuation of octreotide LAR after long term, successful treatment of patients with acromegaly: is it worth trying? Eur. J. Endocrinol. 166, 21–26 (2012).
Ronchi, C. L. et al. Preliminary data on biochemical remission of acromegaly after somatostatin analogs withdrawal. Eur. J. Endocrinol. 158, 19–25 (2008).
Colao, A. et al. Pasireotide LAR is significantly more effective than octreotide LAR at inducing biochemical control in patients with acromegaly: results of a 12-month randomized, double-blind, multicenter, phase III study. Endocrine Abstr. 29, OC1.1 (2012).
Somm, E. et al. A botulinum toxin-derived targeted secretion inhibitor downregulates the GH/IGF1 axis. J. Clin. Invest. 122, 3295–3306 (2012).
Morin, E., Berthelet, F., Weisnagel, J., Bidlingmaier, M. & Serri, O. Failure of temozolomide and conventional doses of pegvisomant to attain biochemical control in a severe case of acromegaly. Pituitary 15, 97–100 (2012).
Hagen, C., Schroeder, H. D., Hansen, S., Hagen, C. & Andersen, M. Temozolomide treatment of a pituitary carcinoma and two pituitary macroadenomas resistant to conventional therapy. Eur. J. Endocrinol. 161, 631–637 (2009).
Acknowledgements
The authors thank all participants in the Ninth Acromegaly Consensus Group meeting: Michel Aubert (Switzerland), John Ayuk (UK), Ariel Barkan (USA), Albert Beckers (Belgium), Paolo Beck-Peccoz (Italy), Anat Ben Shlomo (USA), Nienke Biermasz (Netherlands), Jens Bollerslev (Norway), Philippe Caron (France), Kazuo Chihara (Japan), Annamaria Colao (Italy), Ettore Degli Uberti (Italy), Eleni Dimarki (USA), William Drake (UK), Diego Ferone (Italy), Maria Fleseriu (USA), Hidenori Fukuoka (Japan), Monica Gadelha (Brazil), Yona Greenman (Israel), Ashley Grossman (UK), Peter Kamenicky (France), Larry Katznelson (USA), Marta Korbonits (UK), Andre Lacroix (Canada), Anton Luger (Austria), Gherardo Mazziotti (Italy), Moises Mercado (Mexico), Sebastian Neggers (Netherlands), Vera Popovic-Brkic (Serbia), Johannes Romijn (Netherlands), Jochen Schopohl (Germany), Gunther Stalla (Germany), Peter Trainer (UK), Jacqueline Trouillas (France), Stylianos Tsagarakis (Greece), Mary Lee Vance (USA), John Wass (UK), Susan Webb (Spain), Margaret Wierman (USA) and Whitney Woodmansee (USA). This study was sponsored by an unrestricted educational grant from Ipsen Ltd, Paris, France. Scientific sponsorship of the meeting was provided by Cedars-Sinai Medical Center. We also acknowledge the editorial assistance provided by Martin Gilmour (ESP Bioscience, Crowthorne, UK), who attended the workshop to take minutes that were used to assist the authors in writing.
Author information
Authors and Affiliations
Contributions
S.M. and A.G. wrote the majority of the manuscript. All authors made substantial contribution to discussion of content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.G. has received consultancy fees from Ipsen, Novartis and Pfizer. P.C. has received consultancy fees and research funding from Ipsen, Novartis and Pfizer. D.K. has received research funding from Novartis, and research funding and consulting fees from Eli Lilly. M.D.B. has received consultancy fees from Chiasma, Ipsen and Novartis, received speaking honoraria from Ipsen and Novartis, and is primary investigator on clinical trials sponsored by Ipsen and Novartis. D.C. has received consultancy fees from Ipsen and Pfizer. A.K. has received research funding from Ipsen and Novartis. A.J.v.d.L. has received research funding and consultancy fees from Ipsen, Novartis and Pfizer. C.S. has received consultancy fees or served as an advisory board member for Chiasma, Pfizer, Novartis, Sandoz and Merck Serono, and received speaker honoraria from Ipsen, Pfizer, Novartis, Novo Nordisk and Eli Lilly. K.K.Y.H. has received consultancy fees from Pfizer and Novartis and speaker honoraria from Novartis and Ipsen. F.F.C. has received fees for advisory board participation from Novartis and research funding from Pfizer. S.M. has received consultancy fees from Chiasma, Novartis, Genentech and Ipsen, and research funding from Pfizer and Novartis. S.W.L. declares no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Giustina, A., Chanson, P., Kleinberg, D. et al. Expert consensus document: A consensus on the medical treatment of acromegaly. Nat Rev Endocrinol 10, 243–248 (2014). https://doi.org/10.1038/nrendo.2014.21
Published:
Issue date:
DOI: https://doi.org/10.1038/nrendo.2014.21
This article is cited by
-
Pituitary tumor centers of excellence (PTCOE): the next border of acromegaly treatment
Pituitary (2024)
-
Consensus on criteria for acromegaly diagnosis and remission
Pituitary (2024)
-
Pilot study to define criteria for Pituitary Tumors Centers of Excellence (PTCOE): results of an audit of leading international centers
Pituitary (2023)
-
Two- and three-dimensional endoscopic endonasal surgery of large and giant pituitary adenomas—outcome analysis of a series of 62 patients from a single pituitary center
Neurosurgical Review (2023)
-
Pasireotide effects on biochemical control and glycometabolic profile in acromegaly patients switched from combination therapies or unconventional dosages of somatostatin analogs
Journal of Endocrinological Investigation (2023)