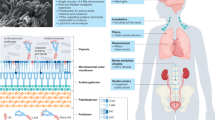
Key Points
-
It was once thought that Mycobacterium tuberculosis in humans arose as a zoonosis, perhaps from Mycobacterium bovis that infected domesticated cattle. Genome sequencing has reversed this view and revealed that M. bovis and related animal ecotypes are likely to be derived from a human-adapted mycobacterium. Moreover, a molecular clock analysis based on sequence from smooth tubercle bacilli and M. tuberculosis has led to the hypothesis that human-infecting M. tuberculosis may have existed as far back as 2.8 million years ago.
-
Whole-genome sequencing (WGS) and analyses of hundreds of M. tuberculosis strains have resulted in a detailed phylogeny and a potential scenario for the emergence of human tuberculosis. According to this scenario, human-infecting mycobacteria arose in Africa and spread with early human migrations out of Africa. Subsequent human population expansion resulted in a concurrent growth of the M. tuberculosis population and its phylogeographical structuring.
-
Unlike most bacteria, M. tuberculosis does not seem to undergo lateral gene transfer or substantial genome rearrangement. Recent reports have documented the first large-scale duplications in M. tuberculosis, all of which affect the same large genomic regions. However, apart from this example, M. tuberculosis seems to evolve primarily through sequential chromosomal mutations.
-
The rate of genome evolution has implications for the ongoing emergence of drug resistance. WGS has been used to establish the mutation rate of M. tuberculosis. Surprisingly, the mutation rate does not seem to differ between active and latent disease. Although the mutation rate for M. tuberculosis is thought to be lower than most bacteria, sequencing of patient isolates has revealed that mutations causing resistance to many drugs can be acquired multiple times by different isolates and even multiple times within the same patient. Moreover, differences in mutation rates between different M. tuberculosis lineages have been linked to differences in the rate of drug resistance acquisition in the laboratory.
-
Although it was once thought that all drug resistance mutations would result in a competitive fitness cost relative to susceptible strains, studies have now demonstrated that clinical strains frequently harbour mutations with low or no fitness cost and that the fitness cost of other mutations can be offset by compensatory mutations.
-
Combining genome sequencing technologies with other whole-cell assay technologies is enabling the study of M. tuberculosis at a comprehensive molecular systems level. A recent systems analysis of the regulatory network of M. tuberculosis on the basis of chromatin immunoprecipitation followed by sequencing (ChIP–seq) revealed complex regulatory interactions that may coordinate different adaptions to the host environment. The data also reveal that transcription factor binding in bacteria is more diverse than previously appreciated.
-
A successful M. tuberculosis infection requires an immune response that is sufficient to establish a granuloma but that is not robust enough to result in sterilization. A emerging picture is that M. tuberculosis has evolved over a long evolutionary history to specifically interact with the human immune system in order to orchestrate this balance. One striking piece of evidence for this is the observation from WGS that antigens in M. tuberculosis are highly conserved.
Abstract
Prevalent since pre-history, human tuberculosis — caused by the pathogen Mycobacterium tuberculosis — remains a major source of death worldwide. Moreover, increasing drug resistance poses the threat of disease resurgence. However, the expanding application of genomic techniques is providing new avenues for combating this old foe. Whole-genome sequencing, comparative genomics and systems biology are generating new insights into the origins and ongoing evolution of M. tuberculosis, as well as the molecular basis for its pathogenicity. These have important implications for our perspective of the disease, development of new drugs and vaccines, and treatment of patients using existing therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
World Health Organization. Global Tuberculosis Control (WHO, 2010).
Russell, D. G. Mycobacterium tuberculosis: here today, and here tomorrow. Nature Rev. Mol. Cell Biol. 2, 569–577 (2001).
Russell, D. G. Who puts the tubercle in tuberculosis? Nature Rev. Microbiol. 5, 39–47 (2006).
Kaufmann, S. H. E. How can immunology contribute to the control of tuberculosis? Nature Rev. Immunol. 1, 20–30 (2001).
Parrish, N. M., Dick, J. D. & Bishai, W. R. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6, 107–112 (1998).
Veyrier, F. J., Dufort, A. & Behr, M. A. The rise and fall of the Mycobacterium tuberculosis genome. Trends Microbiol. 19, 156–161 (2011).
Kapur, V., Whittam, T. S. & Musser, J. M. Is Mycobacterium tuberculosis 15,000 years old? J. Infect. Dis. 170, 1348–1349 (1994).
Bates, J. H. & Stead, W. W. The history of tuberculosis as a global epidemic. Med. Clin. North Am. 77, 1205–1217 (1993).
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998); erratum 396, 190 (1998). This is a groundbreaking report of the first whole-genome sequence for M. tuberculosis.
Behr, M. A. et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284, 1520–1523 (1999).
Gordon, S. V. et al. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32, 643–655 (1999).
Brosch, R. et al. Genomic analysis reveals variation between Mycobacterium tuberculosis H37Rv and the attenuated M. tuberculosis H37Ra strain. Infect. Immun. 67, 5768–5774 (1999).
Brosch, R. et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl Acad. Sci. USA 99, 3684–3689 (2002). This analysis of genomic deletions reveals that M. bovis and related animal-adapted ecotypes are probably derived from human-adapted M. tuberculosis , which reverses the prevailing view that M. tuberculosis is a zoonosis derived from domesticated animals.
Mostowy, S. & Behr, M. A. The origin and evolution of Mycobacterium tuberculosis. Clin. Chest Med. 26, 207–216 (2005).
van Soolingen, D. et al. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int. J. Syst. Bacteriol. 47, 1236–1245 (1997).
Koeck, J. L. et al. Clinical characteristics of the smooth tubercle bacilli 'Mycobacterium canettii' infection suggest the existence of an environmental reservoir. Clin. Microbiol. Infect. 17, 1013–1019 (2011).
Fabre, M. et al. Molecular characteristics of “Mycobacterium canettii” the smooth Mycobacterium tuberculosis bacilli. Infect. Genet. Evol. 10, 1165–1173 (2010).
Gutierrez, M. C. et al. Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. 1, e5 (2005). This is the first report to indicate that STBs are an early branching lineage of the MTBC, which leads to the controversial suggestion that human tuberculosis-causing mycobacteria may have evolved up to 2.8 million years ago.
Supply, P. et al. Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nature Genet. 45, 172–179 (2013).
Gagneux, S. Genetic diversity in Mycobacterium tuberculosis. Curr. Top. Microbiol. Immunol. 374, 1–25 (2013).
Biet, F. et al. Inter- and intra-subtype genotypic differences that differentiate Mycobacterium avium subspecies paratuberculosis strains. BMC Microbiol. 12, 264 (2012).
Turenne, C. Y., Wallace, R. Jr & Behr, M. A. Mycobacterium avium in the postgenomic era. Clin. Microbiol. Rev. 20, 205–229 (2007).
Turenne, C. Y., Collins, D. M., Alexander, D. C. & Behr, M. A. Mycobacterium avium subsp. paratuberculosis and M. avium subsp. avium are independently evolved pathogenic clones of a much broader group of M. avium organisms. J. Bacteriol. 190, 2479–2487 (2008).
Sreevatsan, S. et al. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl Acad. Sci. USA 94, 9869–9874 (1997).
Musser, J. M., Amin, A. & Ramaswamy, S. Negligible genetic diversity of Mycobacterium tuberculosis host immune system protein targets: evidence of limited selective pressure. Genetics 155, 7–16 (2000).
Comas, I. et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nature Genet. 42, 498–503 (2010). This study analyses whole-genome sequences from 21 M. tuberculosis strains and reveals that T cell antigens are as conserved as essential genes. These results suggest that M. tuberculosis antigens may have evolved to specifically interact with the human immune system.
Comas, I. et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nature Genet. 45, 1176–1182 (2013). This analysis of genome sequences from 259 MTBC strains has provided the most complete phylogeny of M. tuberculosis so far and supports a detailed scenario of the origins of human tuberculosis.
Coscolla, M. & Gagneux, S. Does M. tuberculosis genomic diversity explain disease diversity? Drug Discov. Today Dis. Mech. 7, e43–e59 (2010).
Wirth, T. et al. Origin, spread and demography of the Mycobacterium tuberculosis complex. PLoS Pathog. 4, e1000160 (2008).
Brudey, K. et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 6, 23 (2006).
Mathema, B., Kurepina, N. E., Bifani, P. J. & Kreiswirth, B. N. Molecular epidemiology of tuberculosis: current insights. Clin. Microbiol. Rev. 19, 658–685 (2006).
Hershberg, R. et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6, e311 (2008).
Mostowy, S., Cousins, D., Brinkman, J., Aranaz, A. & Behr, M. A. Genomic deletions suggest a phylogeny for the Mycobacterium tuberculosis complex. J. Infect. Dis. 186, 74–80 (2002).
Gagneux, S. & Small, P. M. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect. Dis. 7, 328–337 (2007).
Gagneux, S. et al. Variable host–pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 103, 2869–2873 (2006).
Behr, M. A. Mycobacterium du jour: what's on tomorrow's menu? Microbes Infect. 10, 968–972 (2008).
Firdessa, R. et al. Mycobacterial lineages causing pulmonary and extrapulmonary tuberculosis, Ethiopia. Emerg. Infect. Dis. 19, 460–463 (2013).
Garnier, T. et al. The complete genome sequence of Mycobacterium bovis. Proc. Natl Acad. Sci. USA 100, 7877–7882 (2003).
Prodinger, W. M. et al. Characterization of Mycobacterium caprae isolates from Europe by mycobacterial interspersed repetitive unit genotyping. J. Clin. Microbiol. 43, 4984–4992 (2005).
Cousins, D. V. et al. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int. J. Syst. Evol. Microbiol. 53, 1305–1314 (2003).
Gagneux, S. Host–pathogen coevolution in human tuberculosis. Phil. Trans. R. Soc. B 367, 850–859 (2012).
Pepperell, C. S. et al. The role of selection in shaping diversity of natural M. tuberculosis populations. PLoS Pathog. 9, e1003543 (2013).
Coscolla, M. et al. Novel Mycobacterium tuberculosis complex isolate from a wild chimpanzee. Emerg. Infect. Dis. 19, 969–976 (2013).
Cousins, D. V., Peet, R. L., Gaynor, W. T., Williams, S. N. & Gow, B. L. Tuberculosis in imported hyrax (Procavia capensis) caused by an unusual variant belonging to the Mycobacterium tuberculosis complex. Vet. Microbiol. 42, 135–145 (1994).
Alexander, K. A. et al. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg. Infect. Dis. 16, 1296–1299 (2010).
Udwadia, Z. & Vendoti, D. Totally drug-resistant tuberculosis (TDR-tuberculosis) in India: every dark cloud has a silver lining. J. Epidemiol. Commun. Health 67, 471–472 (2013).
Migliori, G. B., De Iaco, G., Besozzi, G., Centis, R. & Cirillo, D. M. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill 12, E070517.1 (2007).
Klopper, M. et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg. Infect. Dis. 19, 449–455 (2013).
Velayati, A. A. et al. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136, 420–425 (2009).
Ohno, S. Evolution by Gene Duplication (Springer-Verlag, 1970).
Sandegren, L. & Andersson, D. I. Bacterial gene amplification: implications for the evolution of antibiotic resistance. Nature Rev. Microbiol. 7, 578–588 (2009).
Ochman, H., Lawrence, J. G. & Groisman, E. A. Lateral gene transfer and the nature of bacterial innovation. Nature 405, 299–304 (2000).
Boucher, Y. et al. Lateral gene transfer and the origins of prokaryotic groups. Annu. Rev. Genet. 37, 283–328 (2003).
Darling, A. E., Miklos, I. & Ragan, M. A. Dynamics of genome rearrangement in bacterial populations. PLoS Genet. 4, e1000128 (2008).
Kinsella, R. J., Fitzpatrick, D. A., Creevey, C. J. & McInerney, J. O. Fatty acid biosynthesis in Mycobacterium tuberculosis: lateral gene transfer, adaptive evolution, and gene duplication. Proc. Natl Acad. Sci. USA 100, 10320–10325 (2003).
Jang, J., Becq, J., Gicquel, B., Deschavanne, P. & Neyrolles, O. Horizontally acquired genomic islands in the tubercle bacilli. Trends Microbiol. 16, 303–308 (2008).
Becq, J. et al. Contribution of horizontally acquired genomic islands to the evolution of the tubercle bacilli. Mol. Biol. Evol. 24, 1861–1871 (2007).
Stinear, T. P. et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 18, 729–741 (2008).
Veyrier, F. J., Dufort, A. & Behr, M. A. The rise and fall of the Mycobacterium tuberculosis genome. Trends Microbiol. 19, 156–161 (2011).
Supply, P. et al. Linkage disequilibrium between minisatellite loci supports clonal evolution of Mycobacterium tuberculosis in a high tuberculosis incidence area. Mol. Microbiol. 47, 529–538 (2003).
Hirsh, A. E., Tsolaki, A. G., DeRiemer, K., Feldman, M. W. & Small, P. M. Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc. Natl Acad. Sci. USA 101, 4871–4876 (2004).
Namouchi, A., Didelot, X., Schock, U., Gicquel, B. & Rocha, E. P. After the bottleneck: genome-wide diversification of the Mycobacterium tuberculosis complex by mutation, recombination, and natural selection. Genome Res. 22, 721–734 (2012).
Tsolaki, A. G. et al. Functional and evolutionary genomics of Mycobacterium tuberculosis: insights from genomic deletions in 100 strains. Proc. Natl Acad. Sci. USA 101, 4865–4870 (2004).
Ho, T. B., Robertson, B. D., Taylor, G. M., Shaw, R. J. & Young, D. B. Comparison of Mycobacterium tuberculosis genomes reveals frequent deletions in a 20 kb variable region in clinical isolates. Yeast 17, 272–282 (2000).
Sampson, S. L., Richardson, M., Van Helden, P. D. & Warren, R. M. IS6110-mediated deletion polymorphism in isogenic strains of Mycobacterium tuberculosis. J. Clin. Microbiol. 42, 895–898 (2004).
Sampson, S. L. et al. IS6110-mediated deletion polymorphism in the direct repeat region of clinical isolates of Mycobacterium tuberculosis. J. Bacteriol. 185, 2856–2866 (2003).
Alexander, D. C., Turenne, C. Y. & Behr, M. A. Insertion and deletion events that define the pathogen Mycobacterium avium subsp. paratuberculosis. J. Bacteriol. 191, 1018–1025 (2009).
Domenech, P., Kolly, G. S., Leon-Solis, L., Fallow, A. & Reed, M. B. Massive gene duplication event among clinical isolates of the Mycobacterium tuberculosis W/Beijing family. J. Bacteriol. 192, 4562–4570 (2010).
Weiner, B. et al. Independent large scale duplications in multiple M. tuberculosis lineages overlapping the same genomic region. PLoS ONE 7, e26038 (2012).
McDonough, M. A. & Butterton, J. R. Spontaneous tandem amplification and deletion of the shiga toxin operon in Shigella dysenteriae 1. Mol. Microbiol. 34, 1058–1069 (1999).
Bennett, P. M. Genome plasticity: insertion sequence elements, transposons and integrons, and DNA rearrangement. Methods Mol. Biol. 266, 71–113 (2004).
Ford, C. B. et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nature Genet. 43, 482–486 (2011). This WGS analysis of M. tuberculosis Erdman strains that have been recovered from infected cynomolgus macaques was used to estimate the mutation rate of M. tuberculosis in vivo . Surprisingly, the results suggest that the mutation rates are similar during active and latent disease.
Ford, C. B. et al. Mycobacterium tuberculosis mutation rate estimates from different lineages predict substantial differences in the emergence of drug-resistant tuberculosis. Nature Genet. 45, 784–790 (2013). This paper shows that M. tuberculosis strains from lineage 2 have a higher mutation rate and thus acquire drug resistance more rapidly than strains from lineage 4.
McGrath, M., Gey van Pittius, N. C., van Helden, P. D., Warren, R. M. & Warner, D. F. Mutation rate and the emergence of drug resistance in Mycobacterium tuberculosis. J. Antimicrob. Chemother. 69, 292–302 (2013).
Gill, W. P. et al. A replication clock for Mycobacterium tuberculosis. Nature Med. 15, 211–214 (2009).
Munoz-Elias, E. J. et al. Replication dynamics of Mycobacterium tuberculosis in chronically infected mice. Infect. Immun. 73, 546–551 (2005).
Cohen, N. R., Lobritz, M. A. & Collins, J. J. Microbial persistence and the road to drug resistance. Cell Host Microbe 13, 632–642 (2013).
Kohanski, M. A., DePristo, M. A. & Collins, J. J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 37, 311–320 (2010).
Dwyer, D. J., Kohanski, M. A. & Collins, J. J. Role of reactive oxygen species in antibiotic action and resistance. Curr. Opin. Microbiol. 12, 482–489 (2009).
Ioerger, T. R. et al. Genome analysis of multi- and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa. PLoS ONE 4, e7778 (2009).
Ioerger, T. R. et al. The non-clonality of drug resistance in Beijing-genotype isolates of Mycobacterium tuberculosis from the Western Cape of South Africa. BMC Genomics 11, 670 (2010).
Mariam, S. H., Werngren, J., Aronsson, J., Hoffner, S. & Andersson, D. I. Dynamics of antibiotic resistant Mycobacterium tuberculosis during long-term infection and antibiotic treatment. PLoS ONE 6, e21147 (2011).
Casali, N. et al. Microevolution of extensively drug-resistant tuberculosis in Russia. Genome Res. 22, 735–745 (2012).
Sun, G. et al. Dynamic population changes in Mycobacterium tuberculosis during acquisition and fixation of drug resistance in patients. J. Infect. Dis. 206, 1724–1733 (2012). This WGS study of the bacterial population from serial samples from three patients during the acquisition of drug resistance reveals that different resistance mutations can independently arise multiple times in an individual patient.
Fortune, S. M. The surprising diversity of Mycobacterium tuberculosis: change you can believe in. J. Infect. Dis. 206, 1642–1644 (2012).
Nachega, J. B. & Chaisson, R. E. Tuberculosis drug resistance: a global threat. Clin. Infect. Dis. 36, S24–S30 (2003).
Cirz, R. T. & Romesberg, F. E. Induction and inhibition of ciprofloxacin resistance-conferring mutations in hypermutator bacteria. Antimicrob. Agents Chemother. 50, 220–225 (2006).
Boshoff, H. I., Reed, M. B., Barry, C. E., 3rd & Mizrahi, V. DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell 113, 183–193 (2003).
Drobniewski, F. et al. Drug-resistant tuberculosis, clinical virulence, and the dominance of the Beijing strain family in Russia. JAMA 293, 2726–2731 (2005).
Johnson, R. et al. Drug-resistant tuberculosis epidemic in the Western Cape driven by a virulent Beijing genotype strain. Int. J. Tuberc Lung Dis. 14, 119–121 (2010).
Cohen-Bacrie, S. et al. Imported extensively drug-resistant Mycobacterium tuberculosis Beijing genotype, Marseilles, France, 2011. Euro Surveill 16, 19846 (2011).
Kubica, T. et al. The Beijing genotype is a major cause of drug-resistant tuberculosis in Kazakhstan. Int. J. Tuberc Lung Dis. 9, 646–653 (2005).
Mestre, O. et al. Phylogeny of Mycobacterium tuberculosis Beijing strains constructed from polymorphisms in genes involved in DNA replication, recombination and repair. PLoS ONE 6, e16020 (2011).
Muller, B., Borrell, S., Rose, G. & Gagneux, S. The heterogeneous evolution of multidrug-resistant Mycobacterium tuberculosis. Trends Genet. 29, 160–169 (2013).
Gagneux, S. et al. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312, 1944–1946 (2006). This paper shows that clinically derived drug-resistant strains most frequently harbour mutations with small or no fitness cost. This contrasts with laboratory-derived resistant strains, the mutations in which frequently affect competitive fitness.
Billington, O. J., McHugh, T. D. & Gillespie, S. H. Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 43, 1866–1869 (1999).
Davies, A. P. et al. Comparison of fitness of two isolates of Mycobacterium tuberculosis, one of which had developed multi-drug resistance during the course of treatment. J. Infect. 41, 184–187 (2000).
Pym, A. S., Saint-Joanis, B. & Cole, S. T. Effect of katG mutations on the virulence of Mycobacterium tuberculosis and the implication for transmission in humans. Infect. Immun. 70, 4955–4960 (2002).
Sander, P. et al. Fitness cost of chromosomal drug resistance-conferring mutations. Antimicrob. Agents Chemother. 46, 1204–1211 (2002).
Bottger, E. C., Springer, B., Pletschette, M. & Sander, P. Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nature Med. 4, 1343–1344 (1998).
Comas, I. et al. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nature Genet. 44, 106–110 (2011).
de Vos, M. et al. Putative compensatory mutations in the rpoC gene of rifampin-resistant Mycobacterium tuberculosis are associated with ongoing transmission. Antimicrob. Agents Chemother. 57, 827–832 (2013).
Galagan, J. E. et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499, 178–183 (2013). This study presents a comprehensive mapping of the M. tuberculosis regulatory network, which suggests complex regulatory links between processes that are required for adaptations to the host environment. The results also provide the most complete map of transcription factor binding for any bacterium so far and indicate that transcription factor interactions are more diverse than previously thought.
Robertson, G. et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nature Methods 4, 651–657 (2007).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Johnson, D. S., Mortazavi, A., Myers, R. M. & Wold, B. Genome-wide mapping of in vivo protein–DNA interactions. Science 316, 1497–1502 (2007).
Galagan, J., Lyubetskaya, A. & Gomes, A. ChIP–seq and the complexity of bacterial transcriptional regulation. Curr. Top. Microbiol. Immunol. 363, 43–68 (2013).
Jaini, S. et al. Molecular Genetics of Mycobacteria 2nd edn (eds Hatfull, G. & Jacobs, W. R. Jr.) (ASM Press, in the press).
Farnham, P. J. Insights from genomic profiling of transcription factors. Nature Rev. Genet. 10, 605–616 (2009).
Ideker, T. & Sharan, R. Protein networks in disease. Genome Res. 18, 644 (2013).
Alon, U. Network motifs: theory and experimental approaches. Nature Rev. Genet. 8, 450–461 (2007).
Guelzim, N., Bottani, S., Bourgine, P. & Kepes, F. Topological and causal structure of the yeast transcriptional regulatory network. Nature Genet. 31, 60–63 (2002).
Alon, U. An Introduction to Systems Biology: Design Principles of Biological Circuits (Chapman and Hall/CRC, 2006).
Levine, J. H., Lin, Y. & Elowitz, M. B. Functional roles of pulsing in genetic circuits. Science 342, 1193–1200 (2013).
Bennett, M. R. & Hasty, J. Microfluidic devices for measuring gene network dynamics in single cells. Nature Rev. Genet. 10, 628–638 (2009).
Yosef, N. & Regev, A. Impulse control: temporal dynamics in gene transcription. Cell 144, 886–896 (2011).
Wakamoto, Y. et al. Dynamic persistence of antibiotic-stressed mycobacteria. Science 339, 91–95 (2013).
Singh, A. et al. Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog. 5, e1000545 (2009).
Schnappinger, D. et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198, 693–704 (2003).
Rohde, K., Yates, R. M., Purdy, G. E. & Russell, D. G. Mycobacterium tuberculosis and the environment within the phagosome. Immunol. Rev. 219, 37–54 (2007).
Yang, X., Nesbitt, N. M., Dubnau, E., Smith, I. & Sampson, N. S. Cholesterol metabolism increases the metabolic pool of propionate in Mycobacterium tuberculosis. Biochemistry 48, 3819–3821 (2009).
Kendall, S. L. et al. Cholesterol utilization in mycobacteria is controlled by two TetR-type transcriptional regulators: kstR and kstR2. Microbiology 156, 1362–1371 (2010).
Kendall, S. L. et al. A highly conserved transcriptional repressor controls a large regulon involved in lipid degradation in Mycobacterium smegmatis and Mycobacterium tuberculosis. Mol. Microbiol. 65, 684–699 (2007).
Nesbitt, N. M. et al. A thiolase of Mycobacterium tuberculosis is required for virulence and production of androstenedione and androstadienedione from cholesterol. Infect. Immun. 78, 275–282 (2010).
Uhia, I., Galan, B., Medrano, F. J. & Garcia, J. L. Characterization of the KstR-dependent promoter of the first step of cholesterol degradative pathway in Mycobacterium smegmatis. Microbiology 157, 2670–2680 (2011).
Munoz-Elias, E. J. & McKinney, J. D. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nature Med. 11, 638–644 (2005).
Daniel, J., Maamar, H., Deb, C., Sirakova, T. D. & Kolattukudy, P. E. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 7, e1002093 (2011).
Low, K. L. et al. Triacylglycerol utilization is required for regrowth of in vitro hypoxic nonreplicating Mycobacterium bovis bacillus Calmette–Guerin. J. Bacteriol. 191, 5037–5043 (2009).
Peyron, P. et al. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 4, e1000204 (2008). This paper shows that oxygenated mycolic acids from M. tuberculosis are sufficient to induce the differentiation of macrophages into foamy macrophages, which contain lipid bodies that have been shown to be accessible to M. tuberculosis as a potential nutrient source.
Russell, D. G., Cardona, P. J., Kim, M. J., Allain, S. & Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nature Immunol. 10, 943–948 (2009).
Singh, A. et al. Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe–4S] cluster and is essential for nutrient starvation survival. Proc. Natl Acad. Sci. USA 104, 11562–11567 (2007).
Russell, D. G. The evolutionary pressures that have molded Mycobacterium tuberculosis into an infectious adjuvant. Curr. Opin. Microbiol. 16, 78–84 (2013).
Russell, D. G. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol. Rev. 240, 252–268 (2011).
Russell, D. G., Mwandumba, H. C. & Rhoades, E. E. Mycobacterium and the coat of many lipids. J. Cell Biol. 158, 421–426 (2002).
Russell, D. G. et al. Mycobacterium tuberculosis wears what it eats. Cell Host Microbe 8, 68–76 (2010).
Ehlers, S. & Schaible, U. E. The granuloma in tuberculosis: dynamics of a host–pathogen collusion. Front. Immunol. 3, 411 (2012).
Comas, I. & Gagneux, S. A role for systems epidemiology in tuberculosis research. Trends Microbiol. 19, 492–500 (2011).
Pawlowski, A., Jansson, M., Skold, M., Rottenberg, M. E. & Kallenius, G. Tuberculosis and HIV co-infection. PLoS Pathog. 8, e1002464 (2012).
Dooley, K. E. & Chaisson, R. E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 9, 737–746 (2009).
Brothwell, D. R. & Sandison, A. T. Diseases in Antiquity: a Survey of the Diseases, Injuries, and Surgery of Early Populations (C. C. Thomas, 1967).
Prasad, P. V. General medicine in Atharvaveda with special reference to Yaksma (consumption/tuberculosis). Bull. Indian Inst. Hist. Med. Hyderabad 32, 1–14 (2002).
Daniel, V. S. & Daniel, T. M. Old Testament biblical references to tuberculosis. Clin. Infect. Dis. 29, 1557–1558 (1999).
Roberts, C. A. & Buikstra, J. E. The Bioarchaeology of Tuberculosis: a Global View on a Reemerging Disease (Univ. Press of Florida, 2008).
Donoghue, H. D. et al. Tuberculosis: from prehistory to Robert Koch, as revealed by ancient DNA. Lancet Infect. Dis. 4, 584–592 (2004).
Zink, A. et al. Molecular history of tuberculosis from ancient mummies and skeletons. Int. J. Osteoarchaeol. 17, 380 (2007).
Bouwman, A. S. et al. Genotype of a historic strain of Mycobacterium tuberculosis. Proc. Natl Acad. Sci. 109, 18511–18516 (2012).
Zimmerman, M. R. Pulmonary and osseous tuberculosis in an Egyptian mummy. Bull. N. Y. Acad. Med. 55, 604–608 (1979).
Crubezy, E. et al. Identification of Mycobacterium DNA in an Egyptian Pott's disease of 5,400 years old. C. R. Acad. Sci. III 321, 941–951 (1998).
Zink, A., Haas, C. J., Reischl, U., Szeimies, U. & Nerlich, A. G. Molecular analysis of skeletal tuberculosis in an ancient Egyptian population. J. Med. Microbiol. 50, 355–366 (2001).
Zink, A. R., Grabner, W., Reischl, U., Wolf, H. & Nerlich, A. G. Molecular study on human tuberculosis in three geographically distinct and time delineated populations from ancient Egypt. Epidemiol. Infect. 130, 239–249 (2003).
Zink, A. R. et al. Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping. J. Clin. Microbiol. 41, 359–367 (2003).
Nerlich, A. G. & Losch, S. Paleopathology of human tuberculosis and the potential role of climate. Interdiscip Perspect. Infect. Dis. 2009, 437187 (2009).
Nicklisch, N. et al. Rib lesions in skeletons from early neolithic sites in Central Germany: on the trail of tuberculosis at the onset of agriculture. Am. J. Phys. Anthropol. 149, 391–404 (2012).
Formicola, V., Milanesi, Q. & Scarsini, C. Evidence of spinal tuberculosis at the beginning of the fourth millennium BC from Arene Candide cave (Liguria, Italy). Am. J. Phys. Anthropol. 72, 1–6 (1987).
Sager, P., Schalimtzer, M. & Moller-Christensen, V. A case of spondylitis tuberculosa in the Danish Neolithic Age. Dan Med. Bull. 19, 176–180 (1972).
Fusegawa, H. et al. Outbreak of tuberculosis in a 2000-year-old Chinese population. Kansenshogaku Zasshi 77, 146–149 (2003).
Hershkovitz, I. et al. Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS ONE 3, e3426 (2008).
Klaus, H. et al. Tuberculosis on the north coast of Peru: skeletal and molecular paleopathology of late pre-Hispanic and postcontact mycobacterial disease. J. Archaeol. Sci. 37, 2587 (2010).
Salo, W. L., Aufderheide, A. C., Buikstra, J. & Holcomb, T. A. Identification of Mycobacterium tuberculosis DNA in a pre-Columbian Peruvian mummy. Proc. Natl Acad. Sci. USA 91, 2091–2094 (1994).
Arriaza, B. T., Salo, W., Aufderheide, A. C. & Holcomb, T. A. Pre-Columbian tuberculosis in northern Chile: molecular and skeletal evidence. Am. J. Phys. Anthropol. 98, 37–45 (1995).
Rothschild, B. M. & Martin, L. D. Did ice-age bovids spread tuberculosis? Naturwissenschaften 93, 565–569 (2006).
Rothschild, B. M. & Laub, R. Hyperdisease in the late Pleistocene: validation of an early 20th century hypothesis. Naturwissenschaften 93, 557–564 (2006).
Borgdorff, M. W. & van Soolingen, D. The re-emergence of tuberculosis: what have we learnt from molecular epidemiology? Clin. Microbiol. Infect. 19, 889–901 (2013).
Gori, A. et al. Spoligotyping and Mycobacterium tuberculosis. Emerg. Infect. Dis. 11, 1242–1248 (2005).
Gori, A. et al. Comparison between spoligotyping and IS6110 restriction fragment length polymorphisms in molecular genotyping analysis of Mycobacterium tuberculosis strains. Mol. Cell Probes 19, 236–244 (2005).
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Marraffini, L. A. & Sontheimer, E. J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 (2008).
Schurch, A. C. & van Soolingen, D. DNA fingerprinting of Mycobacterium tuberculosis: from phage typing to whole-genome sequencing. Infect. Genet. Evol. 12, 602–609 (2012).
McAdam, R. A. et al. Characterization of a Mycobacterium tuberculosis insertion sequence belonging to the IS3 family. Mol. Microbiol. 4, 1607–1613 (1990).
Coros, A., DeConno, E. & Derbyshire, K. M. IS6110, a Mycobacterium tuberculosis complex-specific insertion sequence, is also present in the genome of Mycobacterium smegmatis, suggestive of lateral gene transfer among mycobacterial species. J. Bacteriol. 190, 3408–3410 (2008).
Supply, P. et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 44, 4498–4510 (2006).
Djelouadji, Z., Arnold, C., Gharbia, S., Raoult, D. & Drancourt, M. Multispacer sequence typing for Mycobacterium tuberculosis genotyping. PLoS ONE 3, e2433 (2008).
Maccallum, I. et al. ALLPATHS 2: small genomes assembled accurately and with high continuity from short paired reads. Genome Biol. 10, R103 (2009).
Butler, J. et al. ALLPATHS: de novo assembly of whole-genome shotgun microreads. Genome Res. 18, 810–820 (2008).
Zerbino, D. R. Using the Velvet de novo assembler for short-read sequencing technologies. Curr. Protoc. Bioinformatics 31, 11.5.1–11.5.12 (2010).
Denisov, G. et al. Consensus generation and variant detection by Celera Assembler. Bioinformatics 24, 1035–1040 (2008).
Zhang, T., Luo, Y., Chen, Y., Li, X. & Yu, J. BIGrat: a repeat resolver for pyrosequencing-based re-sequencing with Newbler. BMC Res. Notes 5, 567 (2012).
Quail, M. A. et al. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics 13, 341 (2012).
Bashir, A. et al. A hybrid approach for the automated finishing of bacterial genomes. Nature Biotech. 30, 701–707 (2012).
Koren, S. et al. Reducing assembly complexity of microbial genomes with single-molecule sequencing. Genome Biol. 14, R101 (2013).
Walker, T. M. et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect. Dis. 13, 137–146 (2013).
Gardy, J. L. et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N. Engl. J. Med. 364, 730–739 (2011).
Bryant, J. M. et al. Whole-genome sequencing to establish relapse or re-infection with Mycobacterium tuberculosis: a retrospective observational study. Lancet Respir. Med. 1, 786–792 (2013).
Acknowledgements
The author thanks E. Azizi, A. Earl and B. Birren for their input and assistance. This project has been funded in whole or in part with Federal funds from the US National Institute of Allergy and Infectious Diseases, US National Institutes of Health, Department of Health and Human Services, under Contract Number HHSN272200800059C, and a grant from the Massachusetts Life Sciences Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Supplementary information
Supplementary information S1 (table)
Reports of archaeological TB samples (PDF 254 kb)
Supplementary information S2 (figure)
Detailed phylogenetic trees. (PDF 219 kb)
Glossary
- Active disease
-
A Mycobacterium tuberculosis infection associated with clinical symptoms of tuberculosis.
- Granuloma
-
An organized nodule of inflammatory cells that is associated with a range of diseases, including tuberculosis. A tuberculosis granuloma consists of a core of infected macrophages surrounded by phagocytes that are enclosed in a mantle of lymphocytes and a fibrous cuff.
- Professional pathogen
-
An organism adapted for a pathogenic lifestyle.
- IS6110 repeat sequence
-
An insertion element specific to the Mycobacterium tuberculosis complex.
- Spoligotyping
-
A PCR-based method for genotyping Mycobacterium tuberculosis strains on the basis of the presence or absence of clustered regularly interspaced short palindromic repeat (CRISPR) spacer sequences.
- D1 deletion
-
A genomic region deleted specifically in Mycobacterium tuberculosis relative to the M. tuberculosis complex.
- Zoonotic transmission
-
The transmission of an animal disease to humans.
- Smooth tubercle bacilli
-
(STBs). Mycobacteria that show a smooth colony morphology on culture media.
- Pulse-field gel electrophoresis
-
A technique for separating large DNA molecules in a gel matrix using a modulating electric field.
- Pre-Columbian
-
Pertaining to the time period before the arrival of Christopher Columbus in the Americas.
- Molecular clock
-
A method for dating divergence between species based on the hypothesis of a constant rate of molecular change over time in selectively neutral DNA sequences.
- Clonal expansion
-
A population that arises from a single cell through cell replication without lateral gene transfer.
- Genetic bottleneck
-
A reduction in genetic diversity of a species as a result of a large decrease in population size.
- Multiple-drug resistant
-
(MDR). Pertaining to tuberculosis that is resistant to at least the first-line drugs isoniazid and rifampicin.
- Extensively drug resistant
-
(XDR). Pertaining to tuberculosis that is resistant to both isoniazid and rifampicin, to a fluoroquinolone and to at least one of the three injectable second-line drugs amikacin, capreomycin or kanamycin.
- Homoplasy
-
Identical character states (for example, the same nucleotide in a DNA sequence) that are not the result of common ancestry (that is, not homologous) but that arose independently in different ancestors by convergent mutations.
- Latent disease
-
A Mycobacterium tuberculosis infection that does not have any clinical symptoms.
- Reactivated disease
-
A Mycobacterium tuberculosis infection that has progressed from latent to active disease.
- Selective sweeps
-
Reductions in genetic variation due to strong selection for advantageous alleles.
- Purifying selection
-
(Also known as negative selection). The removal of deleterious alleles.
Rights and permissions
About this article
Cite this article
Galagan, J. Genomic insights into tuberculosis. Nat Rev Genet 15, 307–320 (2014). https://doi.org/10.1038/nrg3664
Published:
Issue date:
DOI: https://doi.org/10.1038/nrg3664
This article is cited by
-
Will we ever eradicate animal tuberculosis?
Irish Veterinary Journal (2023)
-
Determination of critical concentration for drug susceptibility testing of Mycobacterium tuberculosis against para-aminosalicylic acid with clinical isolates with thyA, folC and dfrA mutations
Annals of Clinical Microbiology and Antimicrobials (2022)
-
The population genomics of within-host Mycobacterium tuberculosis
Heredity (2021)
-
Insights into the evolutionary history of the virulent factor HBHA of Mycobacterium tuberculosis
Archives of Microbiology (2021)
-
Tracing cross species transmission of Mycobacterium bovis at the wildlife/livestock interface in South Africa
BMC Microbiology (2020)