Key Points
-
Lysosomal storage diseases (LSDs) are caused by mutations in genes that encode lysosomal proteins, in most cases lysosomal enzymes.
-
Most treatment strategies for LSDs are based on replacing the missing or defective lysosomal protein in the appropriate target sites of pathology.
-
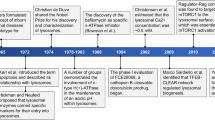
In the 1990s, several 'breakthrough' technologies led to the rapid development of enzyme replacement therapy (ERT) for LSDs. These included: the development of eukaryotic expression systems for lysosomal proteins; and the construction of new mouse models of LSDs by gene targeting.
-
Preclinical studies in animal models have shown the efficacy of ERT for the treatment of LSDs, but have also identified its limitations, including the inability to deliver exogenous enzymes efficiently to the central nervous system (CNS).
-
ERT became available for the first LSD — type I Gaucher disease — in 1991, and ten years of experience in more than 3,000 patients has revealed its remarkable efficacy and safety.
-
ERT is also available for Fabry disease, and is now being evaluated in clinical trials for several other LSDs.
-
New therapeutic strategies, including chaperone-mediated enzyme enhancement therapy (EET), are now being evaluated for LSDs.
-
One principal advantage of EET as compared with ERT is the potential for treating diseases with CNS involvement, because low-molecularweight pharmacological chaperones are able to cross the blood–brain barrier.
Abstract
The past decade has witnessed remarkable advances in our ability to treat inherited metabolic disorders, especially the lysosomal storage diseases, a group of more than 40 disorders, each of which is caused by the deficiency of a lysosomal enzyme or protein. During the past few years, both enzyme replacement and enhancement therapies have been developed to treat these disorders. This review discusses the successes and shortcomings of these therapeutic strategies, and the contributions that they have made to treating lysosomal storage diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Scriver, C. R. et al. (eds) The Metabolic and Molecular Bases of Inherited Diseases (McGraw–Hill, New York, 2001). A four-volume textbook containing chapters on each LSD and other inherited metabolic diseases.
Meikle, P. J., Hopwood, J. J., Clague, A. E. & Carey, W. F. Prevalence of lysosomal storage disorders. JAMA 281, 249–254 (1999).
Holtzman, E. (ed.) Lysosomes (Plenum, New York, 1989).
Kornfeld, S. & Mellman, I. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5, 483–525 (1989).
Sabatini, D. D. & Adesnik, M. B. in The Metabolic and Molecular Bases of Inherited Diseases (eds Scriver, C. R. et al.) 475–484 (McGraw–Hill, New York, 2001).
Kornfeld, S. & Sly, W. S. in The Metabolic and Molecular Bases of Inherited Diseases (eds Scriver, C. R. et al.) 3469–3482 (McGraw–Hill, New York, 2001).
de Duve, C. From cytases to lysosomes. Fed. Proc. 23, 1045 (1964). In this paper, de Duve first proposes that ERT could be used to treat LSDs.
Barton, R. W. & Neufeld, E. F. The Hurler corrective factor. Purification and some properties. J. Biol. Chem. 246, 7773–7779 (1971).
Hickman, S. & Neufeld, E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem. Biophys. Res. Commun. 49, 992–999 (1972).
Kaplan, A., Achord, D. T. & Sly, W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc. Natl Acad. Sci. USA 74, 2026–2030 (1977).
Sahagian, G. G., Distler, J. & Jourdian, G. W. Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular β-galactosidase. Proc. Natl Acad. Sci. USA 78, 4289–4293 (1981).
Kornfeld, S. Lysosomal enzyme targeting. Biochem. Soc. Trans. 18, 367–374 (1990). A seminal review that describes the biosynthesis of N -linked oligosaccharides on lysosomal enzymes, and the M6P-receptor-mediated pathway for the delivery of lysosomal enzymes to the lysosome.
Hille-Rehfeld, A. Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. Biochim. Biophys. Acta 1241, 177–194 (1995).
Kornfeld, S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 61, 307–330 (1992).
Frustaci, A. et al. Improvement in cardiac function in the cardiac variant of Fabry's disease with galactose-infusion therapy. N. Engl. J. Med. 345, 25–32 (2001). First clinical example of EET for an LSD. Administration of galactose as a reversible competitive inhibitor rescued mutant α-galactosidase activity and improved heart function.
Treacy, E. P., Valle, D. & Scriver, C. R. in The Metabolic and Molecular Bases of Inherited Diseases (eds Scriver, C. R. et al.) 175–191 (McGraw–Hill, New York, 2001).
Sly, W. S. & Vogler, C. Brain-directed gene therapy for lysosomal storage disease: going well beyond the blood–brain barrier. Proc. Natl Acad. Sci. USA 99, 5760–5762 (2002).
Cabrera-Salazar, M. A., Novelli, E. & Barranger, J. A. Gene therapy for the lysosomal storage disorders. Curr. Opin. Mol. Ther. 4, 349–358 (2002).
Porter, M. T., Fluharty, A. L. & Kihara, H. Correction of abnormal cerebroside sulfate metabolism in cultured metachromatic leukodystrophy fibroblasts. Science 172, 1263–1265 (1971).
O'Brien, J. S., Miller, A. L., Loverde, A. W. & Veath, M. L. Sanfilippo disease type B: enzyme replacement and metabolic correction in cultured fibroblasts. Science 181, 753–755 (1973).
Cantz, M. & Kresse, H. Sandhoff disease: defective glycosaminoglycan catabolism in cultured fibroblasts and its correction by β-N-acetylhexosaminidase. Eur. J. Biochem. 47, 581–590 (1974).
Desnick, R. J. (ed.) Enzyme Therapy in Genetic Diseases Vol. 2 (Alan R. Liss, Inc., New York, 1980).
Desnick, R. J. & Grabowski, G. in Advances in Human Genetics Vol. 11 (eds Harris, H. & Hirschhorn, K.) 281–349 (Plenum, New York, 1981).
Desnick, R. J. (ed.) Treatment of Genetic Diseases (Churchill Livingstone, New York, 1991).
Johnson, W. G. et al. in Enzyme Therapy in Genetic Diseases (eds Desnick, R. J., Bernlohr, R. W. & Krivit, W.) 120–124 (Williams & Wilkins, Baltimore, Maryland, 1973).
de Barsy, T., Jacquemin, P., van Hoof, F. & Hers, H. G. in Enzyme Therapy in Genetic Diseases (eds Desnick, R. J., Bernlohr, R. W. & Krivit, W.) 184–190 (Williams & Wilkins, Baltimore, Maryland, 1973).
Brady, R. O. et al. Replacement therapy for inherited enzyme deficiency. Use of purified ceramidetrihexosidase in Fabry's disease. N. Engl. J. Med. 289, 9–14 (1973).
Desnick, R. J., Dean, K. J., Grabowski, G., Bishop, D. F. & Sweeley, C. C. Enzyme therapy in Fabry disease: differential in vivo plasma clearance and metabolic effectiveness of plasma and splenic α-galactosidase A isozymes. Proc. Natl Acad. Sci. USA 76, 5326–5330 (1979).
Brady, R. O., Pentchev, P. G., Gal, A. E., Hibbert, S. R. & Dekaban, A. S. Replacement therapy for inherited enzyme deficiency: use of purified glucocerebrosidase in Gaucher's disease. N. Engl. J. Med. 291, 989–993 (1974).
Desnick, R. J., Bernlohr, R. W. & Krivit, W. (eds) Enzyme Therapy in Genetic Diseases (Williams & Wilkins, Baltimore, Maryland, 1973).
von Specht, B. U. et al. Enzyme replacement in Tay–Sachs disease. Neurology 29, 848–854 (1979).
Rattazzi, M. C. & Dobrrenis, K. in Treatment of Genetic Diseases (ed. Desnick, R. J.) 131–152 (Churchill Livingstone, New York, 1991).
Dobrenis, K., Joseph, A. & Rattazzi, M. C. Neuronal lysosomal enzyme replacement using fragment C of tetanus toxin. Proc. Natl Acad. Sci. USA 89, 2297–2301 (1992).
Barton, N. W., Furbish, F. S., Murray, G. J., Garfield, M. & Brady, R. O. Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc. Natl Acad. Sci. USA 87, 1913–1916 (1990). This article convincingly describes the effectiveness of ERT for type I Gaucher disease. These studies provided the proof-of-principle that ERT can reverse the underlying pathology in a human LSD.
Barton, N. W. et al. Replacement therapy for inherited enzyme deficiency — macrophage-targeted glucocerebrosidase for Gaucher's disease. N. Engl. J. Med. 324, 1464–1470 (1991).
Brady, R. O. & Barton, N. W. in Treatment of Genetic Diseases (ed. Desnick, R. J.) 153–168 (Churchill Livingstone, New York, 1991).
Weinreb, N. J. et al. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. Am. J. Med. 113, 112–119 (2002).
Grabowski, G. A., Leslie, N. & Wenstrup, R. Enzyme therapy for Gaucher disease: the first 5 years. Blood Rev. 12, 115–133 (1998).
Mistry, P. K. Gaucher's disease: a model for modern management of a genetic disease. J. Hepatol. 30, 1–5 (1999).
Ioannou, Y. A., Bishop, D. F. & Desnick, R. J. Overexpression of human α-galactosidase A results in its intracellular aggregation, crystallization in lysosomes and selective secretion. J. Cell Biol. 119, 1137–1150 (1992).
Bijvoet, A. G. et al. Human acid α-glucosidase from rabbit milk has therapeutic effect in mice with glycogen storage disease type II. Hum. Mol. Genet. 8, 2145–2153 (1999).
Miranda, S. R. et al. Infusion of recombinant human acid sphingomyelinase into Niemann–Pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 14, 1988–1995 (2000).
Ioannou, Y. A., Zeidner, K. M., Gordon, R. E. & Desnick, R. J. Fabry disease: preclinical studies demonstrate the effectiveness of α-galactosidase A replacement in enzyme-deficient mice. Am. J. Hum. Genet. 68, 14–25 (2001).
Selden, R. F., Skoskiewicz, M. J., Howie, K. B., Russell, P. S. & Goodman, H. M. Implantation of genetically engineered fibroblasts into mice: implications for gene therapy. Science 236, 714–718 (1987).
Cramer, C. L. et al. Bioproduction of human enzymes in transgenic tobacco. Am. NY Acad. Sci. 792, 62–71 (1996).
Desnick, R. J., Patterson, D. F. & Scarpelli, D. G. Animal Models of Inherited Metabolic Diseases (Alan R. Liss, Inc., New York, 1982).
Sango, K. et al. Mouse models of Tay–Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nature Genet. 11, 170–176 (1995).
Platt, F. M. & Butters, T. D. New therapeutic prospects for the glycosphingolipid lysosomal storage diseases. Biochem. Pharmacol. 56, 421–430 (1998).
Wang, A. M. et al. Generation of a mouse model with α-galactosidase A deficiency. Am. J. Hum. Genet. 59, A208 (1996).
Ohshima, T. et al. α-Galactosidase A deficient mice: a model of Fabry disease. Proc. Natl Acad. Sci. USA 94, 2540–2544 (1997).
Horinouchi, K., Sakiyama, T., Pereira, L., Lalley, P. A. & Schuchman, E. H. Mouse models of Niemann–Pick disease: mutation analysis and chromosomal mapping rule out the type A and B forms. Genomics 18, 450–451 (1993).
Otterbach, B. & Stoffel, W. Acid sphingomyelinase-deficient mice mimic the neurovisceral form of human lysosomal storage disease (Niemann–Pick disease). Cell 81, 1053–1061 (1995).
Hess, B. et al. Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc. Natl Acad. Sci. USA 93, 14821–14826 (1996).
Clarke, L. A. et al. Murine mucopolysaccharidosis type I: targeted disruption of the murine α-L-iduronidase gene. Hum. Mol. Genet. 6, 503–511 (1997).
Li, H. H. et al. Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding α-N-acetylglucosaminidase. Proc. Natl Acad. Sci. USA 96, 14505–14510 (1999).
Evers, M. et al. Targeted disruption of the arylsulfatase B gene results in mice resembling the phenotype of mucopolysaccharidosis VI. Proc. Natl Acad. Sci. USA 93, 8214–8219 (1996).
Bijvoet, A. G. et al. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum. Mol. Genet. 7, 53–62 (1998).
Raben, N. et al. Targeted disruption of the acid α-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J. Biol. Chem. 273, 19086–19092 (1998).
Du, H. et al. Enzyme therapy for lysosomal acid lipase deficiency in the mouse. Hum. Mol. Genet. 10, 1639–1648 (2001).
Bhaumik, M. et al. A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome). Glycobiology 9, 1389–1396 (1999).
Vogler, C. et al. A murine model of mucopolysaccharidosis VII. Gross and microscopic findings in β-glucuronidase-deficient mice. Am. J. Pathol. 136, 207–217 (1990).
Tybulewicz, V. L. et al. Animal model of Gaucher's disease from targeted disruption of the mouse glucocerebrosidase gene. Nature 357, 407–410 (1992).
Sidransky, E., Sherer, D. M. & Ginns, E. I. Gaucher disease in the neonate: a distinct Gaucher phenotype is analogous to a mouse model created by targeted disruption of the glucocerebrosidase gene. Pediatr. Res. 32, 494–498 (1992).
Jeyakumar, M. et al. An inducible mouse model of late onset Tay–Sachs disease. Neurobiol. Dis. 10, 201 (2002).
Takahashi, H. et al. Long-term systemic therapy of Fabry disease in a knockout mouse by adeno-associated virus-mediated muscle-directed gene transfer. Proc. Natl Acad. Sci. USA 99, 13777–13782 (2002).
Yuziuk, J. A. et al. Specificity of mouse GM2 activator protein and β-N-acetylhexosaminidases A and B. Similarities and differences with their human counterparts in the catabolism of GM2. J. Biol. Chem. 273, 66–72 (1998).
Brooks, D. A. Immune response to enzyme replacement therapy in lysosomal storage disorder patients and animal models. Mol. Genet. Metab. 68, 268–275 (1999).
Fan, J. Q., Ishii, S., Asano, N. & Suzuki, Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nature Med. 5, 112–115 (1999). The first demonstration of EET for a LSD, in which a substrate inhibitor was used to rescue mutant α-galactosidase A glycopeptides from ER degradation and transport them to the lysosome.
Marathe, S. et al. Creation of a mouse model for non-neurological (type B) Niemann–Pick disease by stable, low level expression of lysosomal sphingomyelinase in the absence of secretory sphingomyelinase: relationship between brain intra-lysosomal enzyme activity and central nervous system function. Hum. Mol. Genet. 9, 1967–1976 (2000).
Altarescu, G. et al. The efficacy of enzyme replacement therapy in patients with chronic neuronopathic Gaucher's disease. J. Pediatr. 138, 539–547 (2001).
Aoki, M. et al. Improvement of neurological symptoms by enzyme replacement therapy for Gaucher disease type IIIb. Eur. J. Pediatr. 160, 63–64 (2001).
Neufeld, E. F. & Muenzer, J. in The Metabolic and Molecular Bases of Inherited Diseases (eds Scriver, C. R. et al.) 3421–3452 (McGraw–Hill, New York, 2001).
Schiffmann, R. et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285, 2743–2749 (2001).
Eng, C. M. et al. Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease. N. Engl. J. Med. 345, 9–16 (2001). This paper reported the results of the largest clinical trial in a LSD, which showed that ERT reversed the fundamental pathology of Fabry disease and was safe and effective for treatment.
Eng, C. M. et al. A phase 1/2 clinical trial of enzyme replacement in Fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am. J. Hum. Genet. 68, 711–722 (2001).
Schiffmann, R. et al. Infusion of α-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease. Proc. Natl Acad. Sci. USA 97, 365–370 (2000).
Van den Hout, H. et al. First clinical test with recombinant human α-glucosidase from rabbit milk shows therapeutic effect in Pompe disease. Am. J. Hum. Genet. 67 (Suppl.2), 6 (2000).
Van den Hout, J. M. et al. Enzyme therapy for Pompe disease with recombinant human α-glucosidase from rabbit milk. J. Inherit. Metab. Dis. 24, 266–274 (2001).
Kishnani, P. et al. Treatment of classical infantile Pompe disease (CIPD) with recombinant human acid α glucosidase (rhGAA): preliminary 6 month data from a phase 2 study. Am. J. Hum. Genet. 71, 582 (2002).
Amalfitano, A. et al. Recombinant human acid α-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet. Med. 3, 132–138 (2001).
van der Ploeg, A. T. et al. Preliminary findings in patients with late onset Pompe's disease treated with recombinant human α-glucosidase from rabbit milk. J. Inherit. Metab. Dis. 25, 118 (2002).
Kakkis, E. D. et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 344, 182–188 (2001).
Beck, M. et al. A phase 3 study of rhIDUA enzyme therapy for MPS I. J. Inherit. Metab. Dis. 25, 120 (2002).
Muenzer, J., Towle, D., Calikoglu, M. & McCandlless, S. A phase I/II clinical study evaluating the safety and clinical activity of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome). Am. J. Hum. Genet. 71, 582 (2002).
Harmatz, P. et al. A phase I/II study of enzyme replacement therapy (ERT) for mucopolysaccharidosis VI (MPS VI; Maroteaux–Lamy syndrome): 48 week progress report. Am. J. Hum. Genet. 71, 582 (2002).
Bonten, E. J. et al. Effective enzyme replacement therapy of murine galactosialidosis using insect cell-expressed PPCA and neuraminidase. Am. J. Hum. Genet. 71, 420 (2002).
Vogler, C. et al. Enzyme replacement in murine mucopolysaccharidosis type VII: neuronal and glial response to β-glucuronidase requires early initiation of enzyme replacement therapy. Pediatr. Res. 45, 838–844 (1999).
Sands, M. S. et al. Biodistribution, kinetics, and efficacy of highly phosphorylated and non-phosphorylated β-glucuronidase in the murine model of mucopolysaccharidosis VII. J. Biol. Chem. 276, 43160–43165 (2001).
Desnick, R. J., Ioannou, Y. A. & Eng, C. M. in The Metabolic and Molecular Bases of Inherited Diseases (eds Scriver, C. R. et al.) 3733–3774 (McGraw–Hill, New York, 2001).
Miranda, S. R., Erlich, S., Friedrich, V. L. Jr, Gatt, S. & Schuchman, E. H. Hematopoietic stem cell gene therapy leads to marked visceral organ improvements and a delayed onset of neurological abnormalities in the acid sphingomyelinase deficient mouse model of Niemann–Pick disease. Gene Ther. 7, 1768–1776 (2000).
Hampton, R. ER-associated degradation in protein quality control and cellular regulation. Curr. Opin. Cell Biol. 14, 476 (2002).
Wagner, E. & Lykke-Andersen, J. mRNA surveillance: the perfect persist. J. Cell Sci. 115, 3033–3038 (2002).
Kerem, B. et al. Identification of the cystic fibrosis gene: genetic analysis. Science 245, 1073–1080 (1989).
Sato, S., Ward, C. L., Krouse, M. E., Wine, J. J. & Kopito, R. R. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J. Biol. Chem. 271, 635–638 (1996).
Cox, D. W. in The Metabolic and Molecular Bases of Inherited Diseases (eds Scriver, C. R. et al.) 5559–5586 (McGraw–Hill, New York, 2001).
Carrell, R. W. & Lomas, D. A. α1-Antitrypsin deficiency — a model for conformational diseases. N. Engl. J. Med. 346, 45–53 (2002).
Loo, T. W. & Clarke, D. M. Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J. Biol. Chem. 272, 709–712 (1997). One of the first descriptions of EET showing that substrates and modulator compounds bound to the multidrug resistance protein could rescue mutant proteins and transport them to the plasma membrane.
Burrows, J. A., Willis, L. K. & Perlmutter, D. H. Chemical chaperones mediate increased secretion of mutant α1-antitrypsin (α1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in α1-AT deficiency. Proc. Natl Acad. Sci. USA 97, 1796–1801 (2000).
Welch, W. J. & Brown, C. R. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1, 109–115 (1996).
Brown, C. R., Hong-Brown, L. Q., Biwersi, J., Verkman, A. S. & Welch, W. J. Chemical chaperones correct the mutant phenotype of the ΔF508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1, 117–125 (1996).
Rubenstein, R. C., Egan, M. E. & Zeitlin, P. L. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing ΔF508-CFTR. J. Clin. Invest. 100, 2457–2465 (1997).
Morello, J. P. et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J. Clin. Invest. 105, 887–895 (2000).
Taylor, J. P., Hardy, J. & Fischbeck, K. H. Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 (2002).
Welch, W. J. & Howard, M. Antagonists to the rescue. J. Clin. Invest. 105, 853–854 (2000).
Morello, J. P., Petaja-Repo, U. E., Bichet, D. G. & Bouvier, M. Pharmacological chaperones: a new twist on receptor folding. Trends Pharmacol. Sci. 21, 466–469 (2000).
Okumiya, T. et al. Galactose stabilizes various missense mutants of α-galactosidase in Fabry disease. Biochem. Biophys. Res. Commun. 214, 1219–1224 (1995).
Asano, N. et al. In vitro inhibition and intracellular enhancement of lysosomal α-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur. J. Biochem. 267, 4179–4186 (2000).
Desnick, R. J., Thorpe, S. R. & Fiddler, M. B. Toward enzyme therapy for lysosomal storage disease. Physiol. Rev. 56, 57–99 (1976).
Doebber, T. W. et al. Enhanced macrophage uptake of synthetically glycosylated human placental β-glucocerebrosidase. J. Biol. Chem. 257, 2193–2199 (1982).
Brady, R. O., Barranger, J. A., Gal, A. E., Pentchev, P. G. & Furbish, F. S. in Enzyme Therapy in Genetic Disease Vol. 2 (ed. Desnick, R. J.) 361–368 (Alan R. Liss, Inc., New York, 1980).
Grabowski, G. A. et al. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann. Intern. Med. 122, 33–39 (1995).
Loldish, H. et al. in Molecular Cell Biology 4th edn, 721 (W. H. Freeman & Co., New York, 2000).
Acknowledgements
The authors thank K. H. Astrin for his assistance with the manuscript. The authors are funded by grants from the National Institutes of Health and a basic research grant from Genzyme Corporation. R.J.D. is a consultant for the Genzyme Corporation and Amicus Therapeutics. E.H.S. is a consultant for the Genzyme Corporation.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
OMIM
Mucopolysaccharidosis type IIIA
Mucopolysaccharidosis type IIIB
Mucopolysaccharidosis type VII
FURTHER INFORMATION
European Agency for Evaluation of Medical Products
Glossary
- SPHINGOLIPID
-
A lipid that contains a sphingosine backbone. Fatty acids are often attached to the nitrogen atom of the sphingosine moiety, generating N-acylsphingosines (ceramides).
- MUCOPOLYSACCHARIDES
-
A group of polysaccharides that have characteristic repeating disaccharide units, each consisting of an N-acetylated hexosamine residue and a uronic-acid residue.
- GLYCOPROTEIN
-
Any one of a class of conjugated proteins that consist of a polypeptide backbone linked to a carbohydrate group.
- ENDOCYTOSIS
-
The uptake of extracellular materials by cells. The plasma membrane invaginates and vesicles pinch off that contain endocytosed molecules and plasma membrane components.
- PHAGOCYTOSIS
-
A process by which cells engulf external soluble and particulate material.
- CATABOLISM
-
A metabolic process by which organisms convert substances into simpler molecules.
- PANCYTOPAENIA
-
A deficiency of all types of blood cell.
- HEPATOSPLENOMEGALY
-
Abnormal enlargement of the spleen and liver.
- NON-NEUTRALIZING ANTIBODY
-
Antibodies that do not inactivate the function of a protein.
- SEROCONVERT
-
The changing of a serological test from negative to positive, indicating the development of antibodies in response to infection or immunization.
- ORPHAN DRUG
-
A drug that is used to treat patients with a rare (or 'orphan') disease.
- GLYCOSPHINGOLIPID
-
A sphingolipid that contains sugars. Common classes of these lipids include cerebrosides and gangliosides.
- PROTEASOME
-
The protein complex that is responsible for degrading intracellular proteins that have been tagged for destruction by the addition of ubiquitin.
- NONSENSE MUTATION
-
A mutation that results in the introduction of a stop codon to cause the premature termination of the protein.
- NONSENSE-MEDIATED MRNA DECAY
-
(NMD). A pathway ensuring that mRNAs that bear premature stop codons are eliminated as templates for translation.
- ACTIVE SITE
-
The part of an enzyme that is responsible for its catalytic function.
- α1-ANTITRYPSIN
-
A serine protease inhibitor that is produced in the liver and secreted into the plasma, which inhibits the activity of trypsin and elastase. α1-Antitrypsin deficiency is associated with emphysema and liver disease.
- OSMOLYTE
-
A molecule that can alter the ionic concentration of a solution.
- P-GLYCOPROTEIN
-
A protein that is responsible for multiple drug resistance.
- GOLGI APPARATUS
-
A lamellar membranous structure, near the nucleus of most cells, that is the site of protein modification (such as N-glycosylation) and sorting.
Rights and permissions
About this article
Cite this article
Desnick, R., Schuchman, E. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet 3, 954–966 (2002). https://doi.org/10.1038/nrg963
Issue date:
DOI: https://doi.org/10.1038/nrg963
This article is cited by
-
Umbilical mesenchymal stem cell-derived extracellular vesicles as enzyme delivery vehicle to treat Morquio A fibroblasts
Stem Cell Research & Therapy (2021)
-
Sphingolipid lysosomal storage diseases: from bench to bedside
Lipids in Health and Disease (2021)
-
Genotype/Phenotype Interactions and First Steps Toward Targeted Therapy for Sphingosine Phosphate Lyase Insufficiency Syndrome
Cell Biochemistry and Biophysics (2021)
-
A DNA nanomachine chemically resolves lysosomes in live cells
Nature Nanotechnology (2019)
-
Targeted enzyme delivery systems in lysosomal disorders: an innovative form of therapy for mucopolysaccharidosis
Cellular and Molecular Life Sciences (2019)