Key Points
-
The lymphatic system is vital for maintaining the colloid osmotic volume (or pressure) and for absorbing lipids from the intestinal tract. The lymphatic system is also essential for the immune response to infectious agents, and during cancer progression; metastatic spreading of malignant cells occurs through the lymphatic and blood vessels.
-
Lymphangiogenesis is the growth of the lymphatic vessels, and congenital or acquired dysfunction of the lymphatic system can result in the formation of lymphoedema — a disorder that results in thickening of the skin and accumulation of adipose tissue.
-
The working model for lymphatic vasculature development proposes that blood venous endothelial cells are the ground condition from which a lymphatic endothelial cell phenotype will be progressively acquired by the stepwise expression of different gene products. In this model, expression of the transcription factor prospero-related homeobox 1 (Prox1) by venous endothelial cells is sufficient to initiate the programme that leads to lymphatic endothelial cell-type specification, and lack of Prox1 activity represses the whole programme of lymphatic differentiation.
-
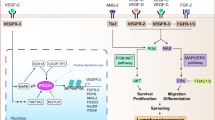
Nonsense mutations in vascular endothelial growth-factor receptor 3 (VEGFR3) have been identified in patients with hereditary lymphoedema and its ligand, VEGFC, was identified as a potent inducer of lymphatic sprouting. Overexpression of VEGFC through the use of a recombinant adenovirus promotes therapeutic lymphangiogenesis in a rabbit model of acquired lymphoedema as well as tumour lymphangiogenesis. Mutations in the forkhead transcription factor FOXC2 have been identified in patients with lymphoedema–distichiasis syndrome.
-
Other available mouse models with lymphatic vasculature alterations include: podoplanin-deficient mice, which have lymphatic defects associated with diminished lymphatic transport, congenital lymphoedema and dilation of lymphatic vessels; neuropilin-2-deficient mice show an absence or severe reduction in the number of small lymphatic vessels and capillaries; and functional inactivation of angiopoietin 2 indicated that this molecule is required for postnatal blood vascular remodelling and proper development of the lymphatic vasculature.
Abstract
Although the process of blood vasculature formation has been well documented, little is known about lymphatic vasculature development, despite its importance in normal and pathological conditions. The lack of specific lymphatic markers has hampered progress in this field. However, the recent identification of genes that participate in the formation of the lymphatic vasculature denotes the beginning of a new era in which better diagnoses and therapeutic treatment(s) of lymphatic disorders could become a reachable goal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
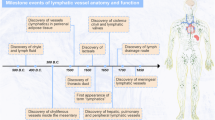
Aselli, G. De Lacteibus sive Lacteis Venis, Quarto Vasorum Mesarai corum Genere novo invento Mediolani, Milano (1627).
Witte, M. H. et al. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc. Res. Tech. 55, 122–145 (2001).
Karpanen, T. et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 61, 1786–1790 (2001).
Mandriota, S. J. et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20, 672–682 (2001).
Skobe, M. et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Med. 7, 192–198 (2001). This paper presents important results about the role of tumour lymphangiogenesis.
Stacker, S. A. et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature Med. 7, 186–191 (2001).
Wigle, J. T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002). The first report that budding venous endothelial cells are a default ground state and after expression of prospero-related homeobox 1 (Prox1) they acquire a lymphatic phenotype.
Oliver, G. & Detmar, M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 16, 773–783 (2002).
Jussila, L. et al. Lymphatic endothelium and Kaposi's sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 58, 1599–1604 (1998).
Skobe, M. et al. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J. Invest. Dermatol. 113, 1047–1053 (1999).
Kampmeier, O. F. Evolution and Comparative Morphology of the Lymphatic System. (Springfield, Illinois, Charles C. Thomas, 1969).
Sabin, F. R. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1, 367–389 (1902). A remarkable, almost accurate, description of the mechanisms involved in lymphatic vasculature development carried out 100 years ago.
Sabin, F. R. On the development of the superficial lymphatics in the skin of the pig. Am. J. Anat. 3, 183–195 (1904).
van der Putte, S. C. The early development of the lymphatic system in mouse embryos. Acta Morphol. Neerl. Scand. 13, 245–286 (1975).
Huntington, G. S. & McClure, C. F. W. The anatomy and development of the jugular lymph sac in the domestic cat (Felis domestica). Am. J. Anat. 10, 177–311 (1910).
Schneider, M., Othman-Hassan, K., Christ, B. & Wilting, J. Lymphangioblasts in the avian wing bud. Dev. Dyn. 216, 311–319 (1999).
Oliver, G. & Harvey, N. A stepwise model of the development of lymphatic vasculature. Ann. NY Acad. Sci. 979, 159–165 (2002).
Grainger, R. M. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet. 8, 349–355 (1992).
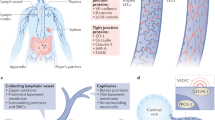
Banerji, S. et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789–801 (1999).
Prevo, R. et al. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 276, 19420–19430 (2001).
Mouta-Carreira, C. et al. LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and downregulation in human liver cancer and cirrhosis. Cancer Res. 61, 8079–8084 (2001).
Knudson, C. B. & Knudson, W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 7, 1233–1241 (1993).
Jackson, D. G., Prevo, R., Clasper, S. & Banerji, S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 22, 317–321 (2001).
Wigle, J. T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999). The first report of a gene product, targeted inactivation of which leads to embryos without any lymphatic vasculature.
Hong, Y. K. et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 225, 351–357 (2002).
Petrova, T. V. et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593–4599 (2002).
Chen, H. et al. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19, 547–559 (1997).
Soker, S. et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735–745 (1998).
Yuan, L. et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 129, 4797–4806 (2002).
Kaipainen, A. et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl Acad. Sci. USA 92, 3566–3570 (1995). The first identification of a gene product with restricted expression in the lymphatic system.
Dumont, D. J. et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282, 946–949 (1998).
Karkkainen, M. J. & Alitalo, K. Lymphatic endothelial regulation, lymphoedema, and lymph node metastasis. Semin. Cell Dev. Biol. 13, 9–18 (2002).
Wetterwald, A. et al. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone 18, 25–32 (1996).
Breiteneder-Geleff, S. et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 154, 385–394 (1999).
Schacht, V. et al. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 22, 3546–3556 (2003).
Partanen, T. A. et al. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 14, 2087–2096 (2000).
Karkkainen, M. J. et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nature Genet. 25, 153–159 (2000). Identification that mutations in vascular endothelial growth-factor receptor 3 ( VEGFR3 ) are responsible for certain forms of hereditary lymphoedema.
Jussila, L. & Alitalo, K. Vascular growth factors and lymphangiogenesis. Physiol. Rev. 82, 673–700 (2002).
Kukk, E. et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development 122, 3829–3837 (1996).
Saaristo, A. et al. Lymphangiogenic gene therapy with minimal blood vascular side effects. J. Exp. Med. 196, 719–730 (2002).
Oh, S. J. et al. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol. 188, 96–109 (1997).
Jeltsch, M. et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276, 1423–1425 (1997).
Enholm, B. et al. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res. 88, 623–629 (2001).
Szuba, A. et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 16, 1985–1987 (2002).
Cao, Y. et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl Acad. Sci. USA 95, 14389–14394 (1998).
Makinen, T. et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 20, 4762–4773 (2001).
Veikkola, T et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 20, 1223–1231 (2001).
Alitalo, K. & Carmeliet, P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 1, 219–227 (2002).
Gale, N. W. et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev. Cell. 3, 411–423 (2002).
Sato, T. N. et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376, 70–74 (1995).
Suri, C. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 (1996).
Abtahian, F. et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299, 247–251 (2003). Intriguing results providing indication of genes and mechanisms that are involved in the separation of the blood and lymphatic vasculature.
Paavonen, K., Puolakkainen, P., Jussila, L., Jahkola, T. & Alitalo, K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am. J. Pathol. 156,1499–1504 (2000).
Milroy, W. F. An undescribed variety of hereditary oedema. NY Med. J. 56, 503 (1892).
Meige, H. Dystophie oedematoeuse hereditaire. Presse Med. 6, 341–343 (1898).
Irrthum, A. et al. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am. J. Hum. Genet. 67, 295–301 (2000).
Ferrell, R. E. et al. Hereditary lymphedema: evidence for linkage and genetic heterogeneity. Hum. Mol. Genet. 7, 2073–2078 (1998).
Karkkainen, M. J. et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl Acad. Sci. USA 98, 12677–12682 (2001). An important demonstration that gene therapy could eventually be achieved in patients with lymphoedema.
Yoon, Y. S. et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J. Clin. Invest. 111, 717–725 (2003).
Fang, J. et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema–distichiasis syndrome. Am. J. Hum. Genet. 67, 1382–1388 (2000). The authors identified forkhead box C2 ( FOXC2 ) as a gene responsible for the hereditary lymphoedema–distichiasis syndrome.
Kriederman, B. M. et al. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema–distichiasis syndrome. Hum. Mol. Genet. 12, 1179–1185 (2003).
Irrthum, A. et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis–lymphedema–telangiectasia. Am. J. Hum. Genet. 72, 1470–1478 (2003).
Mandriota, S. J. et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20, 672–682 (2001).
Beasley, N. J. P. et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 62, 1315–1320 (2002).
Padera, T. P. et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296, 1883–1886 (2002). References 64 and 65 provide antagonistic points of view about the role of intratumoral lymphatics in tumour progression.
Valtola, R. et al. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am. J. Pathol. 154, 1381–1390 (1999).
Lawson, N. D. & Weinstein, B. M. Arteries and veins: making a difference with zebrafish. Nature Rev. Genet. 3, 674–682 (2002).
Gilbert, S. F. Developmental Biology. 7th edn (Sinauer Associates Inc., Massachusetts, 2003).
Szuba, A., Shin, W. S., Strauss, H. W. & Rockson, S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J. Nucl. Med. 44, 43–57 (2003).
Carlson, B. M. Human Embryology and Developmental Biology. 2nd edn (Mosby Inc., USA, 1999).
Stratmann, A., Risau, W. & Plate, K. H. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am. J. Pathol. 153, 1459–1466 (1998).
Zagzag, D. et al. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp. Neurol. 159, 391–400 (1999).
Nibbs, R. J. et al. The β-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am. J. Pathol. 158, 867–877 (2001).
Virata, M. L., Wagner, R. M., Parry, D. A. & Green, K. J. Molecular structure of the human desmoplakin I and II amino terminus. Proc. Natl Acad. Sci. USA 89, 544–548 (1992).
Schmelz, M., Moll, R., Kuhn, C. & Franke, W. W. Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: II. Different types of lymphatic vessels. Differentiation 57, 97–117 (1994).
Ebata, N. et al. Desmoplakin as a specific marker of lymphatic vessels. Microvasc. Res. 61, 40–48 (2001).
Winnier, G. E., Hargett, L. & Hogan, B. L. The winged helix transcription factor MFH1 is required for proliferation and patterning of paraxial mesoderm in the mouse embryo. Genes Dev. 11, 926–940 (1997).
Ayadi, A., Suelves, M., Dolle, P. & Wasylyk, B. Net, an Ets ternary complex transcription factor, is expressed in sites of vasculogenesis, angiogenesis, and chondrogenesis during mouse development. Mech Dev. 102, 205–208 (2001).
Ayadi, A. et al. Net-targeted mutant mice develop a vascular phenotype and upregulate egr-1. EMBO J. 20, 5139–5152 (2001).
Breiteneder-Geleff, S. et al. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is downregulated in puromycin nephrosis. Am. J. Pathol. 151, 1141–1152 (1997).
Wigle, J. T., Chowdhury, K., Gruss, P. & Oliver, G. Prox1 function is crucial for mouse lens-fibre elongation. Nature Genet. 21, 318–322 (1999).
Sosa-Pineda, B., Wigle, J. T. & Oliver, G. Hepatocyte migration during liver development requires Prox1. Nature Genet. 25, 254–255 (2000).
Dyer, M. A., Livesey, F. J., Cepko, C. L. & Oliver, G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nature Genet. 34, 53–58 (2003).
Gunn, M. D. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl Acad. Sci. USA 95, 258–263 (1998).
Gunn, M. D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999).
Witmer, A. N. et al. VEGFR-3 in adult angiogenesis. J. Pathol. 195, 490–497 (2001).
Yoffey, J. M. & Courtice, F. C. Lymphatics, Lymph and the Lymphomyeloid Complex. (Academic Press, London, 1970).
Acknowledgements
I thank J. Wigle and N. Harvey, whose outstanding work in my laboratory has been instrumental in the progress of many of the ideas presented here, J. Morgan and S. Rockson for help with the figures, N. Harvey for critical reading of the manuscript and A. McArthur for scientific editing. This work was supported by the National Institutes of Health, Cancer Center Support from the National Cancer Institute and the American Lebanese Syrian Associated Charities (ALSAC).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
LocusLink
FURTHER INFORMATION
Glossary
- LYMPHANGIOGENESIS
-
The growth of lymphatic vessels.
- LYMPHOEDEMA
-
Occlusion of lymphatic drainage followed by the abnormal accumulation of interstitial fluid and swelling in the affected body part.
- LYMPHATIC METASTASIS
-
Invasion of detaching primary tumour cells through the lymphatic system to lymph nodes. After re-entering of the lymphatic vessels into the blood vasculature, tumour cells will also spread to distant organs.
- PERICYTES
-
Smooth-muscle-like cells that cover the outer surface of the endothelial cells of blood vessels.
- WOLFFIAN BODIES
-
A series of tubules that constitute the mesonephros.
Rights and permissions
About this article
Cite this article
Oliver, G. Lymphatic vasculature development. Nat Rev Immunol 4, 35–45 (2004). https://doi.org/10.1038/nri1258
Issue date:
DOI: https://doi.org/10.1038/nri1258
This article is cited by
-
Understanding the development, pathogenesis, and injury response of meningeal lymphatic networks through the use of animal models
Cellular and Molecular Life Sciences (2023)
-
Disentangling glial diversity in peripheral nerves at single-nuclei resolution
Nature Neuroscience (2022)
-
Podoplanin is Responsible for the Distinct Blood and Lymphatic Capillaries
Cellular and Molecular Bioengineering (2022)
-
Development of Surgical and Visualization Procedures to Analyze Vasculatures by Mouse Tail Edema Model
Biological Procedures Online (2021)
-
Quantitative 3-dimensional imaging and tissue cytometry reveals lymphatic expansion in acute kidney injury
Laboratory Investigation (2021)