Key Points
-
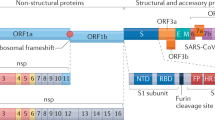
The severe acute respiratory syndrome (SARS), which was first identified in 2003, is caused by a novel coronavirus: the SARS coronavirus (SARS-CoV).
-
Many features of the infection indicate that an excessive, but perhaps 'normal', immune response contributes to SARS.
-
Several coronaviruses cause diseases that result in considerable morbidity and mortality in animals. Some of these diseases are also immune mediated and provide insights into the pathogenesis of SARS.
-
Feline infectious peritonitis virus (FIPV) causes a fatal, immune-mediated disease of felines. Macrophage infection, lymphocyte depletion and antibody-dependent disease enhancement are hallmarks of this disease.
-
Infection with the murine coronavirus murine hepatitis virus (MHV) strain JHM results in immune-mediated demyelination. Similar to SARS, macrophage activation is a key component in the pathogenic process.
-
Another strain of MHV, MHV-3, causes a fatal, fulminant hepatitis. MHV-3 infection of macrophages, with subsequent activation and induction of expression of a novel procoagulant, fibrinogen-like protein 2 (FGL2), is required for severe disease.
-
Chickens that are infected with avian infectious bronchitis virus (IBV) develop respiratory and renal disease. An excessive innate immune response contributes to the pathogenic process in these animals.
-
To develop effective therapies for SARS will require understanding of the contributions of direct injury by virus and of the host immune response to pathogenesis. This requires further studies of the interactions of SARS-CoV with its target cells and necessitates the development of an animal model that reproduces the pulmonary infection that is observed in infected humans.
Abstract
At the end of 2002, the first cases of severe acute respiratory syndrome (SARS) were reported, and in the following year, SARS resulted in considerable mortality and morbidity worldwide. SARS is caused by a novel species of coronavirus (SARS-CoV) and is the most severe coronavirus-mediated human disease that has been described so far. On the basis of similarities with other coronavirus infections, SARS might, in part, be immune mediated. As discussed in this Review, studies of animals that are infected with other coronaviruses indicate that excessive and sometimes dysregulated responses by macrophages and other pro-inflammatory cells might be particularly important in the pathogenesis of disease that is caused by infection with these viruses. It is hoped that lessons from such studies will help us to understand more about the pathogenesis of SARS in humans and to prevent or control outbreaks of SARS in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Griffin, D. E. Immune responses during measles virus infection. Curr. Top. Microbiol. Immunol. 191, 117–134 (1995).
Rehermann, B. & Nascimbeni, M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature Rev. Immunol. 5, 215–229 (2005).
Mejias, A., Chavez-Bueno, S. & Ramilo, O. Respiratory syncytial virus pneumonia: mechanisms of inflammation and prolonged airway hyperresponsiveness. Curr. Opin. Infect. Dis. 18, 199–204 (2005).
Banerjee, S., Narayanan, K., Mizutani, T. & Makino, S. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 76, 5937–5948 (2002).
Menasche, G., Feldmann, J., Fischer, A. & de Saint Basile, G. Primary hemophagocytic syndromes point to a direct link between lymphocyte cytotoxicity and homeostasis. Immunol. Rev. 203, 165–179 (2005).
Parr, R. L. et al. Association of mouse fibrinogen-like protein with murine hepatitis virus-induced prothrombinase activity. J. Virol. 69, 5033–5038 (1995).
Peiris, J. S., Guan, Y. & Yuen, K. Y. Severe acute respiratory syndrome. Nature Med. 10, S88–S97 (2004).
Peiris, J. S., Yuen, K. Y., Osterhaus, A. D. & Stohr, K. The severe acute respiratory syndrome. N. Engl. J. Med. 349, 2431–2441 (2003).
Lai, M. M. C. & Cavanagh, D. The molecular biology of coronaviruses. Adv. Virus Res. 48, 1–100 (1997).
van der Hoek, L. et al. Identification of a new human coronavirus. Nature Med. 10, 368–373 (2004).
Fouchier, R. A. et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl Acad. Sci. USA 101, 6212–6216 (2004).
Woo, P. C. et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79, 884–895 (2005).
Guan, Y. et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302, 276–278 (2003).
Chinese SARS Molecular Epidemiology Consortium. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 303, 1666–1669 (2004). References 13 and 14 trace the evolution of SARS-CoV in human and masked palm-civet populations.
Song, H. D. et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl Acad. Sci. USA 102, 2430–2435 (2005).
Hamming, I. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637 (2004).
Li, W. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003).
Kuba, K. et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nature Med. 11, 875–879 (2005).
Imai, Y. et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436, 112–116 (2005).
Wong, R. S. et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 326, 1358–1362 (2003).
Wang, J. T. et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg. Infect. Dis. 10, 818–824 (2004).
Nicholls, J. M. et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet 361, 1773–1778 (2003).
Chan, K. H. et al. Detection of SARS coronavirus in patients with suspected SARS. Emerg. Infect. Dis. 10, 294–299 (2004).
Peiris, J. S. et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361, 1767–1772 (2003).
Gu, J. et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 202, 415–424 (2005). This is an important histopathological study that indicates that SARS-CoV infection of lymphocytes is a crucial determinant of disease outcome.
Farcas, G. A. et al. Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. J. Infect. Dis. 191, 193–197 (2005).
Mazzulli, T. et al. Severe acute respiratory syndrome-associated coronavirus in lung tissue. Emerg. Infect. Dis. 10, 20–24 (2004).
Lamontagne, L., Descoteaux, J. P. & Jolicoeur, P. Mouse hepatitis virus 3 replication in T and B lymphocytes correlate with viral pathogenicity. J. Immunol. 142, 4458–4465 (1989).
Franks, T. J. et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum. Pathol. 34, 743–748 (2003).
Cheung, C. Y. et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 79, 7819–7826 (2005).
Yilla, M. et al. SARS-coronavirus replication in human peripheral monocytes/macrophages. Virus Res. 107, 93–101 (2005).
Tseng, C. T., Perrone, L. A., Zhu, H., Makino, S. & Peters, C. J. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J. Immunol. 174, 7977–7985 (2005).
Spiegel, M. et al. Inhibition of β interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 79, 2079–2086 (2005). This was the first description of the mechanism of type I IFN suppression of a coronavirus.
Law, H. K. et al. Chemokine upregulation in SARS coronavirus infected human monocyte derived dendritic cells. Blood 106, 2366–2374 (2005).
Wong, C. K. et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136, 95–103 (2004).
Wang, W. K. et al. Temporal relationship of viral load, ribavirin, interleukin (IL)-6, IL-8, and clinical progression in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 39, 1071–1075 (2004).
Zhang, Y. et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect. Immun. 72, 4410–4415 (2004).
Tsui, P. T., Kwok, M. L., Yuen, H. & Lai, S. T. Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg. Infect. Dis. 9, 1064–1069 (2003).
Lee, C. H. et al. Altered p38 mitogen-activated protein kinase expression in different leukocytes with increment of immunosuppressive mediators in patients with severe acute respiratory syndrome. J. Immunol. 172, 7841–7847 (2004).
de Groot, R. J. & Horzinek, M. C. in The Coronaviridae (ed. Siddell, S.) 293–315 (Plenum, New York, 1995).
Stoddart, C. A. & Scott, F. W. Intrinsic resistance of feline peritoneal macrophages to coronavirus infection correlates with in vivo virulence. J. Virol. 63, 436–440 (1989).
de Groot-Mijnes, J. D., van Dun, J. M., van der Most, R. G. & de Groot, R. J. Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease. J. Virol. 79, 1036–1044 (2005). This is a careful study of the clinical and immunological characteristics of FIP.
Hoskins, J. D. Coronavirus infection in cats. Vet. Clin. North Am. Small Anim. Pract. 23, 1–16 (1993).
Tresnan, D. B., Levis, R. & Holmes, K. V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 70, 8669–8674 (1996).
Dean, G. A., Olivry, T., Stanton, C. & Pedersen, N. C. In vivo cytokine response to experimental feline infectious peritonitis virus infection. Vet. Microbiol. 97, 1–12 (2003).
Haagmans, B., Egberink, H. & Horzinek, M. Apoptosis and T-cell depletion during feline infectious peritonitis. J. Virol. 70, 8977–8983 (1996).
Kipar, A. et al. Histopathological alterations of lymphatic tissues in cats without feline infectious peritonitis after long-term exposure to FIP virus. Vet. Microbiol. 69, 131–137 (1999).
Zheng, L. et al. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 377, 348–351 (1995).
Kiss, I., Poland, A. M. & Pedersen, N. C. Disease outcome and cytokine responses in cats immunized with an avirulent feline infectious peritonitis virus (FIPV)-UCD1 and challenge-exposed with virulent FIPV-UCD8. J. Feline Med. Surg. 6, 89–97 (2004).
Kipar, A., Kohler, K., Leukert, W. & Reinacher, M. A comparison of lymphatic tissues from cats with spontaneous feline infectious peritonitis (FIP), cats with FIP virus infection but no FIP, and cats with no infection. J. Comp. Pathol. 125, 182–191 (2001).
Paltrinieri, S., Cammarata, M. P., Cammarata, G. & Comazzi, S. Some aspects of humoral and cellular immunity in naturally occuring feline infectious peritonitis. Vet. Immunol. Immunopathol. 65, 205–220 (1998).
Kipar, A., Bellmann, S., Kremendahl, J., Kohler, K. & Reinacher, M. Cellular composition, coronavirus antigen expression and production of specific antibodies in lesions in feline infectious peritonitis. Vet. Immunol. Immunopathol. 65, 243–257 (1998).
Jacobse-Geels, H. E., Daha, M. R. & Horzinek, M. C. Antibody, immune complexes, and complement activity fluctuations in kittens with experimentally induced feline infectious peritonitis. Am. J. Vet. Res. 43, 666–670 (1982).
Weiss, R. C. & Scott, F. W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp. Immunol. Microbiol. Infect. Dis. 4, 175–189 (1981).
Vennema, H. et al. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. J. Virol. 64, 1407–1409 (1990). This work clearly shows that immunization with vaccinia virus expressing FIPV spike protein results in more severe disease after infection with FIPV.
Lavi, E., Gilden, D., Wroblewska, Z., Rorke, L. & Weiss, S. Experimental demyelination produced by the A59 strain of MHV. Neurology 34, 597–603 (1984).
Lampert, P. W., Sims, J. K. & Kniazeff, A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Acta Neuropathol. 24, 76–85 (1973).
Weiner, L. P. Pathogenesis of demyelination induced by a mouse hepatitis virus (JHM virus). Arch. Neurol. 28, 298–303 (1973).
Stohlman, S. A., Bergmann, C. C. & Perlman, S. in Persistent Viral Infections (eds Ahmed, R. & Chen, I.) 537–557 (Wiley & Sons, New York, 1998).
Perlman, S. Pathogenesis of coronavirus-induced infections: review of pathological and immunological aspects. Adv. Exp. Med. Biol. 440, 503–513 (1998).
Bergmann, C. C. et al. Perforin and γ interferon-mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J. Virol. 78, 1739–1750 (2004).
Wu, G. F. & Perlman, S. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J. Virol. 73, 8771–8780 (1999).
Wang, F., Stohlman, S. A. & Fleming, J. O. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J. Neuroimmunol. 30, 31–41 (1990).
Houtman, J. J. & Fleming, J. O. Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J. Neurovirol. 2, 101–110 (1996). References 61–64, together with reference 72, show that MHV-JHM-induced demyelination is mainly immune mediated.
Lin, M. T., Hinton, D. R., Marten, N. W., Bergmann, C. C. & Stohlman, S. A. Antibody prevents virus reactivation within the central nervous system. J. Immunol. 162, 7358–7368 (1999).
Lin, M. T., Stohlman, S. A. & Hinton, D. R. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J. Virol. 71, 383–391 (1997).
Parra, B. et al. IFN-γ is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162, 1641–1647 (1999).
Fleming, J. O., Trousdale, M. D., El-Zaatari, F., Stohlman, S. A. & Weiner, L. P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J. Virol. 58, 869–875 (1986).
Watanabe, R., Wege, H. & ter Meulen, V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature 305, 150–153 (1983).
Stohlman, S. A. & Hinton, D. R. Viral induced demyelination. Brain Pathol. 11, 92–106 (2001).
Wang, F.-I., Hinton, D., Gilmore, W., Trousdale, M. & Fleming, J. O. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Lab. Invest. 66, 744–754 (1992).
Wu, G. F., Dandekar, A. A., Pewe, L. & Perlman, S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 165, 2278–2286 (2000).
Gombold, J., Sutherland, R., Lavi, E., Paterson, Y. & Weiss, S. R. Mouse hepatitis virus A59-induced demyelination can occur in the absence of CD8+ T cells. Microb. Pathog. 18, 211–221 (1995).
Haring, J. S., Pewe, L. L. & Perlman, S. Bystander CD8 T cell-mediated demyelination after viral infection of the central nervous system. J. Immunol. 169, 1550–1555 (2002).
Kim, T. S. & Perlman, S. Virus-specific antibody, in the absence of T cells, mediates demyelination in mice infected with a neurotropic coronavirus. Am. J. Pathol. 166, 801–809 (2005).
Kim, T. S. & Perlman, S. Viral expression of CCL2 is sufficient to induce demyelination in RAG1−/− mice infected with a neurotropic coronavirus. J. Virol. 79, 7113–7120 (2005).
Pewe, L. L. & Perlman, S. CD8 T cell-mediated demyelination is IFN-γ dependent in mice infected with a neurotropic coronavirus. J. Immunol. 168, 1547–1551 (2002).
Pewe, L., Haring, J. & Perlman, S. CD4 T-cell-mediated demyelination is increased in the absence of γ interferon in mice infected with mouse hepatitis virus. J. Virol. 76, 7329–7333 (2002).
Chen, B. P., Kuziel, W. A. & Lane, T. E. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J. Immunol. 167, 4585–4592 (2001).
Glass, W. G. et al. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J. Immunol. 172, 4018–4025 (2004).
Liu, M. T., Armstrong, D., Hamilton, T. A. & Lane, T. E. Expression of MIG (monokine induced by interferon-γ) is important in T lymphocyte recruitment and host defense following viral infection of the central nervous system. J. Immunol. 166, 1790–1795 (2001).
Liu, M. T. et al. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165, 2327–2330 (2000).
Knobler, R. L., Tunison, L. A. & Oldstone, M. B. Host genetic control of mouse hepatitis virus type 4 (JHM strain) replication. I. Restriction of virus amplification and spread in macrophages from resistant mice. J. Gen. Virol. 65, 1543–1548 (1984).
Xue, S., Sun, N., van Rooijen, N. & Perlman, S. Depletion of blood-borne macrophages does not reduce demyelination in mice infected with a neurotropic coronavirus. J. Virol. 73, 6327–6334 (1999).
Yuwaraj, S., Cattral, M., Pope, M. & Levy, G. Murine hepatitis virus: molecular biology and pathogenesis. Viral Hep. Rev. 2, 125–142 (1996).
Coutelier, J. P. et al. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur. J. Immunol. 24, 1383–1390 (1994).
Pope, M. et al. Pattern of disease after murine hepatitis virus strain 3 infection correlates with macrophage activation and not viral replication. J. Virol. 69, 5252–5260 (1995).
Ding, J. W. et al. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. J. Virol. 71, 9223–9230 (1997).
Marsden, P. A. et al. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. J. Clin. Invest. 112, 58–66 (2003). This work uses FGL2-deficient mice to show the crucial role of this prothrombinase in liver necrosis.
McGilvray, I. D. et al. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J. Biol. Chem. 273, 32222–32229 (1998).
Ning, Q. et al. Induction of prothrombinase fgl2 by the nucleocapsid protein of virulent mouse hepatitis virus is dependent on host hepatic nuclear factor-4α. J. Biol. Chem. 278, 15541–15549 (2003).
Raj, G. D. & Jones, R. C. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 26, 677–706 (1997).
Cavanagh, D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 32, 567–582 (2003).
Hendley, J. O. The host response, not the virus, causes the symptoms of the common cold. Clin. Infect. Dis. 26, 847–848 (1998).
Raj, G. D., Savage, C. E. & Jones, R. C. Effect of heterophil depletion by 5-fluorouracil on infectious bronchitis virus infection in chickens. Avian Pathol. 26, 427–432 (1997).
Chen, B. Y., Hosi, S., Nunoya, T. & Otakura, C. Histopathology and immunochemistry of renal lesions due to infectious bronchitis virus in chickens. Avian Pathol. 25, 269–283 (1996).
Lee, C., Brown, C., Hilt, D. A. & Jackwood, M. W. Nephropathogenesis of chickens experimentally infected with various strains of infectious bronchitis virus. Avian Pathol. 66, 835–840 (2004).
Ware, L. B. & Matthay, M. A. The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 (2000).
Yang, Z. Y. et al. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl Acad. Sci. USA 102, 797–801 (2005).
Subbarao, K. et al. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 78, 3572–3577 (2004).
Martina, B. E. et al. SARS virus infection of cats and ferrets. Nature 425, 915 (2003).
Glass, W. G., Subbarao, K., Murphy, B. & Murphy, P. M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 173, 4030–4039 (2004).
Roberts, A. et al. Severe acute respiratory syndrome coronavirus infection of Golden Syrian hamsters. J. Virol. 79, 503–511 (2005).
Fouchier, R. A. et al. Koch's postulates fulfilled for SARS virus. Nature 423, 240 (2003).
Weingartl, H. et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 78, 12672–12676 (2004).
McAuliffe, J. et al. Replication of SARS coronavirus administered into the respiratory tract of African Green, rhesus and cynomolgus monkeys. Virology 330, 8–15 (2004).
Rowe, T. et al. Macaque model for severe acute respiratory syndrome. J. Virol. 78, 11401–11404 (2004).
Kuiken, T. et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362, 263–270 (2003).
Lassnig, C. et al. Development of a transgenic mouse model susceptible to human coronavirus 229E. Proc. Natl Acad. Sci. USA 102, 8275–8280 (2005).
Li, W. et al. Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J. Virol. 78, 11429–11433 (2004).
Dandekar, A. A., Anghelina, D. & Perlman, S. Bystander CD8 T-cell-mediated demyelination is interferon-γ-dependent in a coronavirus model of multiple sclerosis. Am. J. Pathol. 164, 363–369 (2004).
Goitsuka, R. et al. IL-6 activity in feline infectious peritonitis. J. Immunol. 144, 2599–2603 (1990).
Foley, J. E., Rand, C. & Leutenegger, C. Inflammation and changes in cytokine levels in neurological feline infectious peritonitis. J. Feline Med. Surg. 5, 313–322 (2003).
Garlinghouse, L. E. Jr, Smith, A. L. & Holford, T. The biological relationship of mouse hepatitis virus (MHV) strains and interferon: in vitro induction and sensitivities. Arch. Virol. 82, 19–29 (1984).
Pewe, L. et al. A severe acute respiratory syndrome-associated coronavirus-specific protein enhances virulence of an attenuated murine coronavirus. J. Virol. 79, 11335–11342 (2005).
Acknowledgements
We thank J. Harty, T. Gallagher, S. Varga and N. Butler for comments. This work was supported by the National Institutes of Health (United States) and the National Multiple Sclerosis Society (United States).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- HAEMOPHAGOCYTOSIS
-
The phagocytosis of erythrocytes that results from excessive activation of macrophages. This is usually a consequence of uncontrolled activation and proliferation of T cells.
- MOLECULAR MIMICRY
-
A mechanism for the induction of autoimmunity in which a pathogen expresses a protein or peptide that is similar to a self-protein. After the induction of a pathogen-specific immune response, a crossreactive response to self results in autoimmune pathology.
- EPITOPE SPREADING
-
The de novo activation of autoreactive T cells by self-antigens that have been released after virus-specific T- or B-cell-mediated bystander damage.
- ENZOOTIC VIRUS
-
A virus that infects animals and is endemic to a geographical locale, with only minimal changes in its incidence over time.
- ABORTIVE INFECTION
-
An infection in which viral replication is initiated but no infectious virus is produced.
- SEROSITIS
-
Inflammation of the membranes that line the lungs (the pleura), the heart (the pericardium), and the abdomen (the peritoneum) and the organs within.
- PYOGRANULOMATOUS VASCULITIS
-
A type of vasculitis (that is, inflammation of the blood vessels) that is associated with a chronic inflammatory process in which neutrophils are mixed with components of granulomas.
- ASCITIC FLUID
-
Serous fluid that accumulates in the abdominal cavity. It might result from serositis.
- GRANULOMA
-
A collection of modified macrophages that resemble epithelial cells. This is usually surrounded by a layer of lymphocytes that often includes multinucleated giant cells. Granuloma formation is a chronic inflammatory response that is initiated by various infectious and non-infectious agents.
Rights and permissions
About this article
Cite this article
Dandekar, A., Perlman, S. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol 5, 917–927 (2005). https://doi.org/10.1038/nri1732
Issue date:
DOI: https://doi.org/10.1038/nri1732
This article is cited by
-
Inflammatory cell death, PANoptosis, screen identifies host factors in coronavirus innate immune response as therapeutic targets
Communications Biology (2023)
-
Stem cell therapy: a novel approach against emerging and re-emerging viral infections with special reference to SARS-CoV-2
Molecular Biology Reports (2023)
-
Functional food: complementary to fight against COVID-19
Beni-Suef University Journal of Basic and Applied Sciences (2022)
-
Characteristics of lymphocyte subset alterations in COVID-19 patients with different levels of disease severity
Virology Journal (2022)
-
COVID-19 and cytokine storm syndrome: can what we know about interleukin-6 in ovarian cancer be applied?
Journal of Ovarian Research (2021)