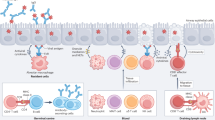
Key Points
-
The increasing number of reports of direct transmission of avian influenza viruses to humans in the past few years and the ongoing outbreak of H5N1 influenza virus infections in birds and humans highlight the pandemic threat posed by avian influenza viruses.
-
Although vaccination is the key strategy for the prevention of severe illness and death from pandemic influenza viruses and despite the long-term experience with vaccines against human influenza viruses, researchers face several obstacles in developing successful vaccines against avian influenza viruses.
-
The haemagglutinin (HA) and neuraminidase (NA) glycoproteins of influenza viruses are the main targets of the protective immune response. Licensed influenza virus vaccines are designed to induce HA-specific antibody responses to protect the host from infection. However, the presence of 16 subtypes of HA and 9 subtypes of NA glycoproteins among avian influenza viruses and the genetic and antigenic diversity among each subtype in nature present several unique challenges for the generation of broadly cross-protective vaccines.
-
Inactivated virus and live attenuated virus vaccines against pandemic influenza are being developed on the basis of plasmid-based reverse-genetics technology. Vaccines based on various other platforms, including live virus vectors and DNA vaccines, are also being developed and show promise in preclinical studies.
-
The available data indicate that inactivated avian influenza virus vaccines are poorly immunogenic and require a high concentration of HA glycoprotein or co-administration with an adjuvant to achieve the desired antibody response in humans. The biological basis for the poor immunogenicity of avian HA glycoproteins is not well understood.
-
Assays to measure the immune response to avian influenza viruses, in particular cell-mediated immune responses, are not available and the immune correlates of protection are not well understood. The choice of assay(s) for assessment of the immune response to pandemic influenza vaccines is a practical challenge in the evaluation of candidate vaccines.
-
As it is difficult to predict which avian influenza virus will cross the species barrier and cause a future pandemic, a library of candidate vaccines of different subtypes must be generated and evaluated in animal models and humans.
-
Although an ideal vaccine would prevent infection, a more realistic goal for a pandemic influenza vaccine might be to prevent severe illness and death.
Abstract
The increasing number of reports of direct transmission of avian influenza viruses to humans underscores the need for control strategies to prevent an influenza pandemic. Vaccination is the key strategy to prevent severe illness and death from pandemic influenza. Despite long-term experience with vaccines against human influenza viruses, researchers face several additional challenges in developing human vaccines against avian influenza viruses. In this Review, we discuss the features of avian influenza viruses, the gaps in our understanding of infections caused by these viruses in humans and of the immune response to them that distinguishes them from human influenza viruses, and the current status of vaccine development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Morens, D. M., Folkers, G. K. & Fauci, A. S. The challenge of emerging and re-emerging infectious diseases. Nature 430, 242–249 (2004).
Crawford, P. C. et al. Transmission of equine influenza virus to dogs. Science 310, 482–485 (2005).
Wright, P. F. & Webster, R. G. in Fields Virology 4th edn (eds Knipe, D. M. & Howley, P. M.) 1533–1579 (Lippincott Williams & Wilkins, Philadelphia, 2001).
Fouchier, R. A. et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 79, 2814–2822 (2005).
Treanor, J. J., Tierney, E. L., Zebedee, S. L., Lamb, R. A. & Murphy, B. R. Passively transferred monoclonal antibody to the M2 protein inhibits influenza A virus replication in mice. J. Virol. 64, 1375–1377 (1990).
Zebedee, S. L. & Lamb, R. A. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J. Virol. 62, 2762–2772 (1988).
Webster, R. G., Bean, W. J., Gorman, O. T., Chambers, T. M. & Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56, 152–179 (1992).
Skehel, J. J. & Wiley, D. C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69, 531–569 (2000).
Perdue, M. L., Garcia, M., Senne, D. & Fraire, M. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res. 49, 173–186 (1997).
Klenk, H. D. & Garten, W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 2, 39–43 (1994).
Kawaoka, Y., Naeve, C. W. & Webster, R. G. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology 139, 303–316 (1984).
Banks, J., Speidel, E. C., McCauley, J. W. & Alexander, D. J. Phylogenetic analysis of H7 haemagglutinin subtype influenza A viruses. Arch. Virol. 145, 1047–1058 (2000).
Akey, B. L. Low-pathogenicity H7N2 avian influenza outbreak in Virginia during 2002. Avian Dis. 47, 1099–1103 (2003).
Marangon, S. & Capua, I. Control of avian influenza in Italy: from stamping out to emergency and prophylactic vaccination. Dev. Biol. (Basel) 124, 109–115 (2006).
Connor, R. J., Kawaoka, Y., Webster, R. G. & Paulson, J. C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 205, 17–23 (1994).
Shinya, K. et al. Avian flu: influenza virus receptors in the human airway. Nature 440, 435–436 (2006).
van Riel, D. et al. H5N1 virus attachment to lower respiratory tract. Science 312, 399 (2006).
Claas, E. C. et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351, 472–477 (1998).
Subbarao, K. et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279, 393–396 (1998). This paper reported the characterization of the H5N1 virus isolated from the index case of human infection with H5N1 avian influenza virus in 1997 in Hong Kong, and showed that an avian H5N1 virus could transmit directly to humans.
Gillim-Ross, L. & Subbarao, K. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 19, 614–636 (2006).
Lewis, D. B. Avian flu to human influenza. Annu. Rev. Med. 57, 139–154 (2006).
Stephenson, I., Nicholson, K. G., Wood, J. M., Zambon, M. C. & Katz, J. M. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect. Dis. 4, 499–509 (2004).
Beigel, J. H. et al. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353, 1374–1385 (2005). A Review summarizing the clinical and laboratory aspects of H5N1 infections in humans in several Asian countries.
de Jong, M. D. & Hien, T. T. Avian influenza A (H5N1). J. Clin. Virol. 35, 2–13 (2006).
Couch, R. B. & Kasel, J. A. Immunity to influenza in man. Annu. Rev. Microbiol. 37, 529–549 (1983). This Review summarizes the importance of antibodies specific for HA and NA glycoproteins in providing protection against influenza virus infections in humans.
Potter, C. W. & Oxford, J. S. Determinants of immunity to influenza infection in man. Br. Med. Bull. 35, 69–75 (1979).
Gerhard, W. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 260, 171–190 (2001).
Kilbourne, E. D., Laver, W. G., Schulman, J. L. & Webster, R. G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J. Virol. 2, 281–288 (1968).
Murphy, B. R., Kasel, J. A. & Chanock, R. M. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N. Engl. J. Med. 286, 1329–1332 (1972).
Choi, Y. K. et al. Continuing evolution of H9N2 influenza viruses in Southeastern China. J. Virol. 78, 8609–8614 (2004).
Fouchier, R. A. et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl Acad. Sci. USA 101, 1356–1361 (2004).
Tweed, S. A. et al. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 10, 2196–2199 (2004).
Suarez, D. L. et al. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg. Infect. Dis. 10, 693–699 (2004).
Russell, R. J. et al. H1 and H7 influenza haemagglutinin structures extend a structural classification of haemagglutinin subtypes. Virology 325, 287–296 (2004). This study describes the genetic basis for the classification of the 15 HA glycoprotein subtypes into four clades.
Makarova, N. V., Kaverin, N. V., Krauss, S., Senne, D. & Webster, R. G. Transmission of Eurasian avian H2 influenza virus to shorebirds in North America. J. Gen. Virol. 80, 3167–3171 (1999).
Cox, N. J. & Bender, C. A. The molecular epidemiology of influenza virus. Semin. Virol. 6, 359–370 (1995).
Wilson, I. A. & Cox, N. J. Structural basis of immune recognition of influenza virus hemagglutinin. Annu. Rev. Immunol. 8, 737–771 (1990).
Lee, C. W., Senne, D. A. & Suarez, D. L. Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J. Virol. 78, 8372–8381 (2004). This study provides evidence for the evolution of the HA glycoprotein of avian influenza viruses when veterinary vaccines are used to prevent avian influenza outbreaks in poultry.
Ghendon, Y. Z. et al. Development of cell culture (MDCK) live cold-adapted (CA) attenuated influenza vaccine. Vaccine 23, 4678–4684 (2005).
Oxford, J. S. et al. A new European perspective of influenza pandemic planning with a particular focus on the role of mammalian cell culture vaccines. Vaccine 23, 5440–5449 (2005).
Palker, T. et al. Protective efficacy of intranasal cold-adapted influenza A/New Caledonia/20/99 (H1N1) vaccines comprised of egg- or cell culture-derived reassortants. Virus Res. 105, 183–194 (2004).
Lu, X. et al. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73, 5903–5911 (1999).
Wood, J. M. et al. Vaccines against H5N1 influenza. Vaccine 18, 579–580 (1999).
Li, S. et al. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J. Infect. Dis. 179, 1132–1138 (1999).
Subbarao, K. et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology 305, 192–200 (2003).
Fodor, E. et al. Rescue of influenza A virus from recombinant DNA. J. Virol. 73, 9679–9682 (1999).
Hoffmann, E., Krauss, S., Perez, D., Webby, R. & Webster, R. G. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine 20, 3165–3170 (2002).
Neumann, G. et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl Acad. Sci. USA 96, 9345–9350 (1999). References 46–48 describe plasmid-based reverse-genetic systems for the generation of recombinant influenza viruses.
Ottolini, M. G. et al. The cotton rat provides a useful small-animal model for the study of influenza virus pathogenesis. J. Gen. Virol. 86, 2823–2830 (2005).
Straight, T. M., Ottolini, M. G., Prince, G. A. & Eichelberger, M. C. Evidence of a cross-protective immune response to influenza A in the cotton rat model. Vaccine 24, 6264–6271 (2006).
Lowen, A. C., Mubareka, S., Tumpey, T. M., Garcia-Sastre, A. & Palese, P. The guinea pig as a transmission model for human influenza viruses. Proc. Natl Acad. Sci. USA 103, 9988–9992 (2006).
Chen, H. et al. Generation and evaluation of a high-growth reassortant H9N2 influenza A virus as a pandemic vaccine candidate. Vaccine 21, 1974–1979 (2003).
Lu, X. et al. Immunity to influenza A H9N2 viruses induced by infection and vaccination. J. Virol. 75, 4896–4901 (2001).
Takada, A. et al. Avirulent avian influenza virus as a vaccine strain against a potential human pandemic. J. Virol. 73, 8303–8307 (1999).
Govorkova, E. A., Webby, R. J., Humberd, J., Seiler, J. P. & Webster, R. G. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J. Infect. Dis. 194, 159–167 (2006).
Lipatov, A. S., Hoffmann, E., Salomon, R., Yen, H. L. & Webster, R. G. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97 (H5) vaccines against infection with A/Vietnam/1203/04 (H5N1) virus in ferrets. J. Infect. Dis. 194, 1040–1043 (2006).
Nicolson, C., Major, D., Wood, J. M. & Robertson, J. S. Generation of influenza vaccine viruses on Vero cells by reverse genetics: an H5N1 candidate vaccine strain produced under a quality system. Vaccine 23, 2943–2952 (2005).
Webby, R. J. et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet 363, 1099–1103 (2004).
Nicholson, K. G. et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 357, 1937–1943 (2001).
Stephenson, I. et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 21, 1687–1693 (2003).
Atmar, R. L. et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin. Infect. Dis. 43, 1135–1142 (2006).
Bresson, J. L. et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367, 1657–1664 (2006).
Hehme, N., Engelmann, H., Kunzel, W., Neumeier, E. & Sanger, R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med. Microbiol. Immunol. (Berlin) 191, 203–208 (2002).
Lin, J. et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet 368, 991–997 (2006).
Wright, P. F. et al. Trials of influenza A/New Jersey/76 virus vaccine in normal children: an overview of age-related antigenicity and reactogenicity. J. Infect. Dis. 136, S731–S741 (1977).
Stephenson, I. et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet 362, 1959–1966 (2003).
Parkman, P. D., Hopps, H. E., Rastogi, S. C. & Meyer, H. M. Jr. Summary of clinical trials of influenza virus vaccines in adults. J. Infect. Dis. 136, S722–S730 (1977).
Ozaki, H. et al. Generation of high-yielding influenza A viruses in African green monkey kidney (Vero) cells by reverse genetics. J. Virol. 78, 1851–1857 (2004).
Treanor, J. J. et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 19, 1732–1737 (2001).
Treanor, J. J., Campbell, J. D., Zangwill, K. M., Rowe, T. & Wolff, M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 354, 1343–1351 (2006).
Maassab, H. F. & Bryant, M. L. The development of live attenuated cold-adapted influenza virus vaccine for humans. Rev. Med. Virol. 9, 237–244 (1999).
Jin, H. et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 306, 18–24 (2003).
Cha, T. A. et al. Genotypic stability of cold-adapted influenza virus vaccine in an efficacy clinical trial. J. Clin. Microbiol. 38, 839–845 (2000).
Chen, H. et al. Generation and characterization of a cold-adapted influenza A H9N2 reassortant as a live pandemic influenza virus vaccine candidate. Vaccine 21, 4430–4436 (2003).
Suguitan, A. L. et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 3, e360 (2006).
Luke, C. J. & Subbarao, K. Vaccines for pandemic influenza. Emerg. Infect. Dis. 12, 66–72 (2006).
Nwe, N. et al. Expression of hemagglutinin protein from the avian influenza virus H5N1 in a baculovirus/insect cell system significantly enhanced by suspension culture. BMC Microbiol. 6, 16 (2006).
de Wit, E. et al. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J. Virol. 79, 12401–12407 (2005).
Ernst, W. A. et al. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine 24, 5158–5168 (2006).
Rimmelzwaan, G. F., Claas, E. C., van Amerongen, G., de Jong, J. C. & Osterhaus, A. D. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine 17, 1355–1358 (1999).
Bright, R. A., Ross, T. M., Subbarao, K., Robinson, H. L. & Katz, J. M. Impact of glycosylation on the immunogenicity of a DNA-based influenza H5 HA glycoprotein vaccine. Virology 308, 270–278 (2003).
Epstein, S. L. et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg. Infect. Dis. 8, 796–801 (2002).
Kodihalli, S., Kobasa, D. L. & Webster, R. G. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine 18, 2592–2599 (2000).
Qiu, M. et al. Protection against avian influenza H9N2 virus challenge by immunization with hemagglutinin- or neuraminidase-expressing DNA in BALB/c mice. Biochem. Biophys. Res. Commun. 343, 1124–1131 (2006).
Epstein, S. L. et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine 23, 5404–5410 (2005).
Gao, W. et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 80, 1959–1964 (2006).
Hoelscher, M. A. et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 367, 475–481 (2006).
Gerhard, W., Mozdzanowska, K. & Zharikova, D. Prospects for universal influenza virus vaccine. Emerg. Infect. Dis. 12, 569–574 (2006).
Zharikova, D., Mozdzanowska, K., Feng, J., Zhang, M. & Gerhard, W. Influenza type A virus escape mutants emerge in vivo in the presence of antibodies to the ectodomain of matrix protein 2. J. Virol. 79, 6644–6654 (2005).
Palese, P. Making better influenza virus vaccines? Emerg. Infect. Dis. 12, 61–65 (2006).
Chen, J., Fang, F., Li, X., Chang, H. & Chen, Z. Protection against influenza virus infection in BALB/c mice immunized with a single dose of neuraminidase-expressing DNAs by electroporation. Vaccine 23, 4322–4328 (2005).
Kilbourne, E. D. et al. Protection of mice with recombinant influenza virus neuraminidase. J. Infect. Dis. 189, 459–461 (2004).
Subbarao, K., Murphy, B. R. & Fauci, A. S. Development of effective vaccines against pandemic influenza. Immunity 24, 5–9 (2006).
Li, Z. N. et al. Chimeric influenza virus hemagglutinin proteins containing large domains of the Bacillus anthracis protective antigen: protein characterization, incorporation into infectious influenza viruses, and antigenicity. J. Virol. 79, 10003–10012 (2005).
Stephenson, I., Wood, J. M., Nicholson, K. G., Charlett, A. & Zambon, M. C. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 103, 91–95 (2004). This study describes the use of horse erythrocytes in a haemagglutination-inhibition assay to improve the sensitivity of detection of antibodies specific for H5N1 avian influenza viruses.
Stephenson, I., Wood, J. M., Nicholson, K. G. & Zambon, M. C. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J. Med. Virol. 70, 391–398 (2003).
Hobson, D., Curry, R. L., Beare, A. S. & Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (London) 70, 767–777 (1972). This study indicates that serum haemagglutination-inhibition antibody titres are a correlate of protection in influenza virus infection of humans.
Treanor, J. & Wright, P. F. Immune correlates of protection against influenza in the human challenge model. Dev. Biol. (Basel) 115, 97–104 (2003).
Rowe, T. et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37, 937–943 (1999). This paper describes the development of a neutralization assay to detect antibodies specific for H5N1 avian influenza virus and records the titre of antibody in convalescent patients.
Stephenson, I. H5N1 vaccines: how prepared are we for a pandemic? Lancet 368, 965–966 (2006).
Johnson, N. P. & Mueller, J. Updating the accounts: global mortality of the 1918–1920 'Spanish' influenza pandemic. Bull. Hist. Med. 76, 105–115 (2002).
Reid, A. H. & Taubenberger, J. K. The origin of the 1918 pandemic influenza virus: a continuing enigma. J. Gen. Virol. 84, 2285–2292 (2003).
Kobasa, D. et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 431, 703–707 (2004).
Tumpey, T. M. et al. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc. Natl Acad. Sci. USA 101, 3166–3171 (2004).
Tumpey, T. M. et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310, 77–80 (2005).
Gamblin, S. J. et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 303, 1838–1842 (2004).
Kawaoka, Y., Krauss, S. & Webster, R. G. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 63, 4603–4608 (1989).
Scholtissek, C., Rohde, W., Von Hoyningen, V. & Rott, R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 87, 13–20 (1978).
Proceedings of the 98th Annual Meeting of the United States Animal Health Association, Grand Rapids, Michigan (1994).
Hatta, M., Gao, P., Halfmann, P. & Kawaoka, Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293, 1840–1842 (2001).
Castrucci, M. R. & Kawaoka, Y. Biologic importance of neuraminidase stalk length in influenza A virus. J. Virol. 67, 759–764 (1993).
Matrosovich, M., Zhou, N., Kawaoka, Y. & Webster, R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 73, 1146–1155 (1999).
Subbarao, E. K., London, W. & Murphy, B. R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 67, 1761–1764 (1993).
Guan, Y., Shortridge, K. F., Krauss, S. & Webster, R. G. Molecular characterization of H9N2 influenza viruses: were they the donors of the 'internal' genes of H5N1 viruses in Hong Kong? Proc. Natl Acad. Sci. USA 96, 9363–9367 (1999).
Hoffmann, E et al. Characterization of the influenza A virus gene pool in avian species in southern China: was H6N1 a derivative or a precursor of H5N1? J. Virol. 74, 6309–6315 (2000).
Lin, Y. P. et al. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc. Natl Acad. Sci. USA 97, 9654–9658 (2000).
Xu, X., Subbarao, K., Cox, N. J. & Guo, Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreak in Hong Kong. Virology 261, 15–19 (1999).
Apisarnthanarak, A. et al. Atypical avian influenza (H5N1). Emerg. Infect. Dis. 10, 1321–1324 (2004).
de Jong, M. D. et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 352, 686–691 (2005).
Tran, T. H. et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350, 1179–1188 (2004).
Epidemic and Pandemic Alert and Response (EPR): Avian influenza. World Health Organisation [online]
Koopmans, M. et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363, 587–593 (2004).
Hirst, M. et al. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg. Infect. Dis. 10, 2192–2195 (2004).
Matrosovich, M. N., Krauss, S. & Webster, R. G. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281, 156–162 (2001).
Guo, Y. J. et al. Characterization of the pathogenicity of members of the newly established H9N2 influenza virus lineages in Asia. Virology 267, 279–288 (2000).
Butt, K. M. et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J. Clin. Microbiol. 43, 5760–5767 (2005).
Avian influenza virus A (H10N7) circulating among humans in Egypt. EID Weekly Updates [online], (2004).
Stephenson, I et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J. Infect. Dis. 191, 1210–1215 (2005).
Pushko, P. et al. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 23, 5751–5759 (2005).
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Kanta Subbarao and Tomy Joseph
Scientific barriers to developing vaccines against avian influenza viruses. Nature Reviews Immunology, published online 16 March 2007; doi: 10.1038/nri2054.
The laboratory of Dr Subbarao has a cooperative research and development agreement with MedImmune Vaccines to develop vaccines against potential pandemic strains of influenza.
Glossary
- Pandemic influenza virus
-
An influenza virus of a new subtype to which the general population has little or no immunity that causes disease in humans and spreads efficiently from person to person, causing community-wide outbreaks and resulting in a global outbreak of influenza.
- Haemagglutinin
-
A type I integral membrane glycoprotein that binds to cell-surface receptors and facilitates fusion between the viral envelope and endosomal membrane. It is the main target antigen of the humoral immune response to influenza viruses.
- Neuraminidase
-
A type II integral membrane glycoprotein that facilitates virus release from cells by removing sialic acid from sialyloligosaccharides on the cell and viral surfaces. It is also a target of the protective immune response.
- Antigenic drift
-
A process by which circulating influenza viruses are constantly changing, which allows the virus to cause annual epidemics of illness. Antigenic drift occurs when mutations accumulate in the haemagglutinin and neuraminidase genes that alter the antigenicity of these proteins such that the 'drifted' strains are no longer neutralized by antibodies that were specific for previously circulating strains.
- Antigenic shift
-
A process by which a new influenza A virus haemagglutinin subtype (with or without an accompanying new neuraminidase subtype) is introduced into the human population, which lacks prior experience of and immunity to the subtype. Antigenic shift can occur as a result of the direct introduction of an influenza virus from an animal or avian host into humans or by the exchange or reassortment of gene segments between human and non-human influenza viruses when they co-infect animals or humans.
- Matrix protein
-
The most abundant structural protein of influenza virus, which lies beneath the virus envelope.
- Nucleoprotein
-
Encapsidates viral genomic RNA and forms a ribonucleoprotein complex in association with viral polymerase proteins.
- Positive immune selection
-
This is usually defined as a significant excess of non-silent over silent nucleotide substitutions in a gene, and occurs when natural selection favours a particular genetic variation and therefore the frequency of the genetic variation shifts.
- Vaccine seed virus
-
A virus that is used for the large-scale production of vaccines.
- PR8 H1N1 influenza virus (A/Puerto Rico/8/34)
-
A well-characterized laboratory strain of influenza virus that confers high growth in eggs and is used as the genetic backbone for viruses from which inactivated influenza virus vaccines are generated.
- Adjuvant
-
An agent mixed with an antigen that increases the immune response to that antigen after immunization.
- Subvirion vaccine
-
In a subvirion vaccine, the virions are disrupted or split by detergent treatment and the surface glycoproteins are then partially purified.
- Cold-adapted virus
-
A virus that replicates efficiently at low temperatures, which can be generated by serial passage of a wild-type virus at successively lower temperatures.
- Haemagglutination-inhibition assay
-
An assay used to measure the concentration of antibodies that inhibit the agglutination of erythrocytes by a standard amount of influenza virus. Serum haemagglutination-inhibition antibody titres correlate with protection from infection with human influenza viruses.
Rights and permissions
About this article
Cite this article
Subbarao, K., Joseph, T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol 7, 267–278 (2007). https://doi.org/10.1038/nri2054
Published:
Issue date:
DOI: https://doi.org/10.1038/nri2054
This article is cited by
-
The generation of hemagglutinin monoclonal antibodies against H9N2 influenza virus
Animal Diseases (2023)
-
The antibody landscapes following AS03 and MF59 adjuvanted H5N1 vaccination
npj Vaccines (2022)
-
Screening and purification of NanB sialidase from Pasteurella multocida with activity in hydrolyzing sialic acid Neu5Acα(2–6)Gal and Neu5Acα(2–3)Gal
Scientific Reports (2022)
-
A single dose of a vesicular stomatitis virus-based influenza vaccine confers rapid protection against H5 viruses from different clades
npj Vaccines (2020)
-
Synthesis of Chalcone Derivatives Containing Furan or/and Pyran Ring as Neuraminidase Inhibitors
Chemical Research in Chinese Universities (2019)