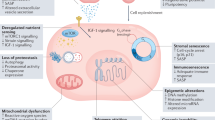
Abstract
The population of elderly individuals with rheumatoid arthritis (RA) is expanding, due mainly to increasing life expectancy. A variety of theories have been proposed to explain the ageing process, including accumulation of DNA damage and resultant changes in biological processes. Such changes can influence the development and/or course of disease. Furthermore, alterations in biological function determine the biological age—as opposed to chronological age—of an individual, which strongly influences their ability to cope with disease. Moreover, comorbidities are more frequent in elderly individuals. Together, these factors complicate treatment of disease and necessitate careful patient management. Indeed, although evidence from clinical trials suggests that DMARDs and biologic agents have good efficacy and are well tolerated in elderly patients with RA, such individuals are often undertreated and inadequately managed. Unfortunately, insufficient data are available for the development of evidence-based guidelines for this population, as elderly patients are often excluded from clinical trials owing to age restrictions or comorbidities. Thus, additional clinical studies in elderly patients are warranted, with treatment regimens tailored according to vitality or frailty parameters. This Review focuses on the pathophysiological aspects of ageing and their implications for the management of RA in elderly patients.
Key Points
-
As a consequence of increasing life expectancies worldwide, the number of elderly people with rheumatoid arthritis (RA) is growing
-
Evidence-based guidelines for management of RA in elderly individuals are lacking owing to insufficient clinical data
-
Data suggest that elderly individuals with RA are undertreated and inadequately managed, despite DMARDs and biologic therapies being effective and seemingly well tolerated in this population
-
Therapeutic decision making in elderly patients with RA needs to incorporate consideration of patient frailty and comorbidities
-
The level of disease activity set as the therapeutic goal might differ according to the patient's biological age or presence of particular risk factors for infection
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Konrat, C. et al. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. PLoS ONE 7, e33559 (2012).
Macieira-Coelho, A. Cell division and aging of the organism. Biogerontology 12, 503–515 (2011).
Sperka, T., Wang, J. & Rudolph, K. L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell. Biol. 13, 579–590 (2012).
Kirkwood, T. B. Evolution of ageing. Nature 270 301–304 (1977).
Kirkwood, T. L., Kapahi, P. & Shanley, D. P. Evolution, stress, and longevity. J. Anat. 197, 587–590 (2000).
Ljubuncic, P. & Reznick, A. Z. The evolutionary theories of aging revisited—a mini-review. Gerontology 55, 205–216 (2009).
Fulop, T. et al. Aging, frailty and age-related diseases. Biogerontology 11, 547–563 (2010).
Isaacs, B. The challenge of geriatric medicine (Oxford University Press, 1997).
Health Council of the Netherlands. Health care for the elderly with multimorbidity. Publication no. 2008/01 (Health Council of the Netherlands, 2008).
Lang, P. O., Michel, J. P. & Zekry, D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 55, 539–549 (2009).
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–156 (2001).
Bandeen-Roche, K. et al. Phenotype of frailty: characterization in the women's health and aging studies. J. Gerontol. A Biol. Sci. Med. Sci. 61, 262–266 (2006).
Garonzik-Wang, J. M. et al. Frailty and delayed graft function in kidney transplant recipients. Arch. Surg. 147, 190–193 (2012).
Makary, M. A. et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 210, 901–908 (2010).
Yao, X. et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 29, 5015–5021 (2011).
Abadir, P. M. et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc. Natl Acad. Sci. USA 108, 14849–14854 (2011).
Leng, S. X., Yang, H. & Walston, J. D. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin. Exp. Res. 16, 249–252 (2004).
Varadhan, R. et al. Higher levels and blunted diurnal variation of cortisol in frail older women. J. Gerontol. A Biol. Sci. Med. Sci. 63, 190–195 (2008).
Gameiro, C. & Romao, F. Changes in the immune system during menopause and aging. Front. Biosci. (Elite Ed.) 2, 1299–1303 (2010).
Gomez, C. R., Boehmer, E. D. & Kovacs, E. J. The aging innate immune system. Curr. Opin. Immunol. 17, 457–462 (2005).
Krabbe, K. S., Pedersen, M. & Bruunsgaard, H. Inflammatory mediators in the elderly. Exp. Gerontol. 39, 687–699 (2004).
Panda, A. et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 30, 325–333 (2009).
Shaw, A. C., Joshi, S., Greenwood, H., Panda, A. & Lord, J. M. Aging of the innate immune system. Curr. Opin. Immunol. 22, 507–513 (2010).
Goronzy, J. J., Shao, L. & Weyand, C. M. Immune aging and rheumatoid arthritis. Rheum. Dis. Clin. North Am. 36, 297–310 (2010).
Lindstrom, T. M. & Robinson, W. H. Rheumatoid arthritis: a role for immunosenescence? J. Am. Geriatr. Soc. 58, 1565–1575 (2010).
Scullion, J. Tuberculosis and older people. Nurs. Older People 15, 23–27 (2003).
Frasca, D. & Blomberg, B. B. Aging affects human B cell responses. J. Clin. Immunol. 31, 430–435 (2011).
Lee, N., Shin, M. S. & Kang, I. T-cell biology in aging, with a focus on lung disease. J. Gerontol. A Biol. Sci. Med. Sci. 67, 254–263 (2012).
Hakim, F. T. et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J. Clin. Invest. 115, 930–939 (2005).
Geginat, J., Lanzavecchia, A. & Sallusto, F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 101, 4260–4266 (2003).
Broux, B., Markovic-Plese, S., Stinissen, P. & Hellings, N. Pathogenic features of CD4+CD28− T cells in immune disorders. Trends Mol. Med. 18, 446–453 (2012).
Weng, N. P., Akbar, A. N. & Goronzy, J. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 30, 306–312 (2009).
Vallejo, A. N. et al. Molecular basis for the loss of CD28 expression in senescent T cells. J. Biol. Chem. 277, 46940–46949 (2002).
Valenzuela, H. F. & Effros, R. B. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin. Immunol. 105, 117–125 (2002).
Koch, S. et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann. NY Acad. Sci. 1114, 23–35 (2007).
van Leeuwen, E. M. et al. Emergence of a CD4+CD28− granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J. Immunol. 173, 1834–1841 (2004).
Schirmer, M., Vallejo, A. N., Weyand, C. M. & Goronzy, J. J. Resistance to apoptosis and elevated expression of Bcl-2 in clonally expanded CD4+CD28− T cells from rheumatoid arthritis patients. J. Immunol. 161, 1018–1025 (1998).
Vallejo, A. N., Schirmer, M., Weyand, C. M. & Goronzy, J. J. Clonality and longevity of CD4+CD28null T cells are associated with defects in apoptotic pathways. J. Immunol. 165, 6301–6307 (2000).
Vallejo, A. N. et al. Expansions of NK-like αβT cells with chronologic aging: novel lymphocyte effectors that compensate for functional deficits of conventional NK cells and T cells. Ageing Res. Rev. 10, 354–361 (2011).
Heyn, H. et al. Distinct DNA methylomes of newborns and centenarians. Proc. Natl Acad. Sci. USA 109, 10522–10527 (2012).
Liu, Y., Chen, Y. & Richardson, B. Decreased DNA methyltransferase levels contribute to abnormal gene expression in “senescent” CD4+CD28− T cells. Clin. Immunol. 132, 257–265 (2009).
Schmidt, D., Martens, P. B., Weyand, C. M. & Goronzy, J. J. The repertoire of CD4+ CD28− T cells in rheumatoid arthritis. Mol. Med. 2, 608–618 (1996).
Schmidt, D., Goronzy, J. J. & Weyand, C. M. CD4+CD7−CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Invest. 97, 2027–2037 (1996).
Bristol-Myers Squibb. Abatacept summary of product characteristics [online]
Kremer, J. M. et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N. Engl. J. Med. 349, 1907–1915 (2003).
Arai, Y., Takayama, M., Abe, Y. & Hirose, N. Adipokines and aging. J. Atheroscler. Thromb. 18, 545–550 (2011).
Conde, J. et al. Adipokines: novel players in rheumatic diseases. Discov. Med. 15, 73–83 (2013).
Gomez, R. et al. What's new in our understanding of the role of adipokines in rheumatic diseases? Nat. Rev. Rheumatol. 7, 528–536 (2011).
Olama, S. M., Senna, M. K. & Elarman, M. Synovial/serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol. Int. 32, 683–690 (2012).
Matarese, G., Procaccini, C., De Rosa, V., Horvath, T. L. & La Cava, A. Regulatory T cells in obesity: the leptin connection. Trends Mol. Med. 16, 247–256 (2010).
Giles, J. T., Allison, M., Bingham, C. O. 3rd, Scott, W. M. Jr & Bathon, J. M. Adiponectin is a mediator of the inverse association of adiposity with radiographic damage in rheumatoid arthritis. Arthritis Rheum. 61, 1248–1256 (2009).
Matthews, S. J. & Lancaster, J. W. Urinary tract infections in the elderly population. Am. J. Geriatr. Pharmacother. 9, 286–309 (2011).
Meyer, K. C. The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly. Semin. Respir. Crit. Care Med. 31, 561–574 (2010).
Mori, T. & Leung, C. C. Tuberculosis in the global aging population. Infect. Dis. Clin. North Am. 24, 751–768 (2010).
Jefferson, T. et al. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 366, 1165–1174 (2005).
Kumar, R. & Burns, E. A. Age-related decline in immunity: implications for vaccine responsiveness. Expert. Rev. Vaccines 7, 467–479 (2008).
Lang, P. O., Govind, S., Mitchell, W. A., Siegrist, C. A. & Aspinall, R. Vaccine effectiveness in older individuals: what has been learned from the influenza-vaccine experience. Ageing Res. Rev. 10, 389–395 (2011).
Lang, P. O. et al. Effectiveness of influenza vaccine in aging and older adults: comprehensive analysis of the evidence. Clin. Interv. Aging 7, 55–64 (2012).
McElhaney, J. E. Influenza vaccine responses in older adults. Ageing Res. Rev. 10, 379–388 (2011).
Fujihashi, K. & Kiyono, H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol. 30, 334–343 (2009).
Giuliano, S., Ohanna, M., Ballotti, R. & Bertolotto, C. Advances in melanoma senescence and potential clinical application. Pigment Cell Melanoma Res. 24, 295–308 (2011).
Lasithiotakis, K. G., Petrakis, I. E. & Garbe, C. Cutaneous melanoma in the elderly: epidemiology, prognosis and treatment. Melanoma Res. 20, 163–170 (2010).
Finkel, T., Serrano, M. & Blasco, M. A. The common biology of cancer and ageing. Nature 448, 767–774 (2007).
de Vlam, K. et al. Detection and identification of antinuclear autoantibodies in the serum of normal blood donors. Clin. Exp. Rheumatol. 11, 393–397 (1993).
Borchers, A. T. & Gershwin, M. E. Giant cell arteritis: a review of classification, pathophysiology, geoepidemiology and treatment. Autoimmun. Rev. 11, A544–A554 (2012).
Crowson, C. S. et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 63, 633–639 (2011).
Fujii, H., Shao, L., Colmegna, I., Goronzy, J. J. & Weyand, C. M. Telomerase insufficiency in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 106, 4360–4365 (2009).
Weyand, C. M., Fujii, H., Shao, L. & Goronzy, J. J. Rejuvenating the immune system in rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 583–588 (2009).
Koetz, K. et al. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl Acad. Sci. USA 97, 9203–9208 (2000).
Goronzy, J. J. & Weyand, C. M. Immune aging and autoimmunity. Cell. Mol. Life Sci. 69, 1615–1623 (2012).
Colmegna, I. et al. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 58, 990–1000 (2008).
Shao, L. et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J. Exp. Med. 206, 1435–1449 (2009).
Kohler, S. & Thiel, A. Life after the thymus: CD31+ and CD31− human naive CD4+ T-cell subsets. Blood 113, 769–774 (2009).
Lee, W. W., Yang, Z. Z., Li, G., Weyand, C. M. & Goronzy, J. J. Unchecked CD70 expression on T cells lowers threshold for T cell activation in rheumatoid arthritis. J. Immunol. 179, 2609–2615 (2007).
Singh, K., Colmegna, I., He, X., Weyand, C. M. & Goronzy, J. J. Synoviocyte stimulation by the LFA-1–intercellular adhesion molecule-2–Ezrin–Akt pathway in rheumatoid arthritis. J. Immunol. 180, 1971–1978 (2008).
Mavragani, C. P. & Moutsopoulos, H. M. Rheumatoid arthritis in the elderly. Exp. Gerontol. 34, 463–471 (1999).
Tutuncu, Z. & Kavanaugh, A. Rheumatic disease in the elderly: rheumatoid arthritis. Clin. Geriatr. Med. 21, 513–525 (2005).
van Schaardenburg, D. & Breedveld, F. C. Elderly-onset rheumatoid arthritis. Semin. Arthritis Rheum. 23, 367–378 (1994).
Villa-Blanco, J. I. & Calvo-Alen, J. Elderly onset rheumatoid arthritis: differential diagnosis and choice of first-line and subsequent therapy. Drugs Aging 26, 739–750 (2009).
Kermani, T. A. & Warrington, K. J. Polymyalgia rheumatica. Lancet 381, 63–72 (2013).
Chen, D. Y. et al. Proinflammatory cytokine profiles of patients with elderly-onset rheumatoid arthritis: a comparison with younger-onset disease. Gerontology 55, 250–258 (2009).
Glennas, A., Kvien, T. K., Andrup, O., Karstensen, B. & Munthe, E. Recent onset arthritis in the elderly: a 5 year longitudinal observational study. J. Rheumatol. 27, 101–108 (2000).
van der Heijde, D. M. et al. Older versus younger onset rheumatoid arthritis: results at onset and after 2 years of a prospective followup study of early rheumatoid arthritis. J. Rheumatol. 18, 1285–1289 (1991).
Gabriel, S. E. & Michaud, K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res. Ther. 11, 229 (2009).
Maradit-Kremers, H. et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 52, 402–411 (2005).
Maradit-Kremers, H., Nicola, P. J., Crowson, C. S., Ballman, K. V. & Gabriel, S. E. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 52, 722–732 (2005).
Nicola, P. J. et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 52, 412–420 (2005).
Madjid, M. & Willerson, J. T. Inflammatory markers in coronary heart disease. Br. Med. Bull. 100, 23–38 (2011).
Liao, K. P. & Solomon, D. H. Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology (Oxford) 52, 45–52 (2013).
Smitten, A. L., Simon, T. A., Hochberg, M. C. & Suissa, S. A meta-analysis of the incidence of malignancy in adult patients with rheumatoid arthritis. Arthritis Res. Ther. 10, R45 (2008).
Baecklund, E. et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 54, 692–701 (2006).
Niccoli, T. & Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 22, R741–R752 (2012).
Askling, J. & Bongartz, T. Malignancy and biologic therapy in rheumatoid arthritis. Curr. Opin. Rheumatol. 20, 334–339 (2008).
Doran, M. F., Crowson, C. S., Pond, G. R., O'Fallon, W. M. & Gabriel, S. E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 46, 2287–2293 (2002).
De Keyser, F. Choice of biologic therapy for patients with rheumatoid arthritis: the infection perspective. Curr. Rheumatol. Rev. 7, 77–87 (2011).
Salliot, C., Dougados, M. & Gossec, L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann. Rheum. Dis. 68, 25–32 (2009).
Crowson, C. S., Hoganson, D. D., Fitz-Gibbon, P. D. & Matteson, E. L. Development and validation of a risk score for serious infection in patients with rheumatoid arthritis. Arthritis Rheum. 64, 2847–2855 (2012).
Seitz, C. S., Berens, N., Brocker, E. B. & Trautmann, A. Leg ulceration in rheumatoid arthritis—an underreported multicausal complication with considerable morbidity: analysis of thirty-six patients and review of the literature. Dermatology 220, 268–273 (2010).
Summers, G. D., Deighton, C. M., Rennie, M. J. & Booth, A. H. Rheumatoid cachexia: a clinical perspective. Rheumatology (Oxford) 47, 1124–1131 (2008).
Munro, R. & Capell, H. Prevalence of low body mass in rheumatoid arthritis: association with the acute phase response. Ann. Rheum. Dis. 56, 326–329 (1997).
Chen, Y. M., Chen, L. K., Lan, J. L. & Chen, D. Y. Geriatric syndromes in elderly patients with rheumatoid arthritis. Rheumatology (Oxford) 48, 1261–1264 (2009).
Ziade, N., Jougla, E. & Coste, J. Population-level influence of rheumatoid arthritis on mortality and recent trends: a multiple cause-of-death analysis in France, 1970–2002. J. Rheumatol. 35, 1950–1957 (2008).
Fraenkel, L., Rabidou, N. & Dhar, R. Are rheumatologists' treatment decisions influenced by patients' age? Rheumatology (Oxford) 45, 1555–1557 (2006).
Schmajuk, G. et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 57, 928–934 (2007).
Radovits, B. J., Fransen, J., Eijsbouts, A., van Riel, P. L. & Laan, R. F. Missed opportunities in the treatment of elderly patients with rheumatoid arthritis. Rheumatology (Oxford) 48, 906–910 (2009).
Radovits, B. J. et al. Influence of age on the outcome of antitumour necrosis factor alpha therapy in rheumatoid arthritis. Ann. Rheum. Dis. 68, 1470–1473 (2009).
Bathon, J. M. et al. Safety and efficacy of etanercept treatment in elderly subjects with rheumatoid arthritis. J. Rheumatol. 33, 234–243 (2006).
Fleischmann, R. M. et al. Response to etanercept (Enbrel) in elderly patients with rheumatoid arthritis: a retrospective analysis of clinical trial results. J. Rheumatol. 30, 691–696 (2003).
Lurati, A., Marrazza, M., Angela, K. & Scarpellini, M. Safety of etanercept in elderly subjects with rheumatoid arthritis. Biologics 4, 1–4 (2010).
Genevay, S. et al. Tolerance and effectiveness of anti-tumor necrosis factor alpha therapies in elderly patients with rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 57, 679–685 (2007).
Galloway, J. B. et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50, 124–131 (2011).
Schneeweiss, S. et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 56, 1754–1764 (2007).
Dotan, E., Browner, I., Hurria, A. & Denlinger, C. Challenges in the management of older patients with colon cancer. J. Natl Compr. Canc. Netw. 10, 213–224 (2012).
Pallis, A. G. & Scarci, M. Are we treating enough elderly patients with early stage non-small cell lung cancer? Lung Cancer 74, 149–154 (2011).
Acknowledgements
The inspiration for this article came from the Academy of Immunology for Clinicians, Belgium, Spring Lecture Sessions on ageing and immune-mediated inflammatory disorders. The authors thank H. Van de Keere (10A, Maldegem, Belgium) and P. Leventhal (4Clinics, Paris, France) for English-language editorial assistance.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussion of content and review/editing of the manuscript before submission. F. De Keyser wrote the article.
Corresponding author
Ethics declarations
Competing interests
P. Masson declares that he has received speakers fees from Abbott (Abbvie); R. Lories declares that he has received speakers or consultancy fees from Abbott (Abbvie), Boehringer Ingelheim, Celgene, Jansen, Merck and Pfizer, and has received research support from Abbott, Celgene, and Pfizer. F. De Keyser declares that he has received consultancy fees from Abbott (Abbvie), AstraZeneca, GSK, MSD, Pfizer and Roche. A. M. H. Boots, A. B. Maier and P. Stinissen declare no competing interests.
Rights and permissions
About this article
Cite this article
Boots, A., Maier, A., Stinissen, P. et al. The influence of ageing on the development and management of rheumatoid arthritis. Nat Rev Rheumatol 9, 604–613 (2013). https://doi.org/10.1038/nrrheum.2013.92
Published:
Issue date:
DOI: https://doi.org/10.1038/nrrheum.2013.92
This article is cited by
-
Long-term effects of abatacept on atherosclerosis and arthritis in older vs. younger patients with rheumatoid arthritis: 3-year results of a prospective, multicenter, observational study
Arthritis Research & Therapy (2024)
-
Prevention of Rheumatoid Arthritis in At-Risk Individuals: Current Status and Future Prospects
Drugs (2024)
-
Age-related self-DNA accumulation may accelerate arthritis in rats and in human rheumatoid arthritis
Nature Communications (2023)
-
Late-onset rheumatoid arthritis registry study, LORIS study: study protocol and design
BMC Rheumatology (2022)
-
Age-related mechanisms in the context of rheumatic disease
Nature Reviews Rheumatology (2022)