Abstract
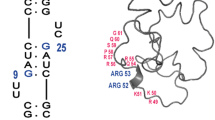
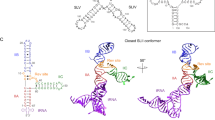
We have used NMR spectroscopy to determine the solution structure of a complex between an oligonucleotide derived from stem IIB of the Rev responsive element (RRE-IIB) of HIV-1 mRNA and an in vivo selected, high affinity binding Arg-rich peptide. The peptide binds in a partially α-helical conformation into a pocket within the RNA deep groove. Comparison with the structure of a complex between an α-helical Rev peptide and RRE-IIB reveals that the sequence of the bound peptide determines the local conformation of the RRE peptide binding site. A conformational switch of an unpaired uridine base was revealed; this points out into the solvent in the Rev peptide complex, but it is stabilized inside the RNA deep groove by stacking with an Arg side chain in the selected peptide complex. The conformational switch has been visualized by NMR chemical shift mapping of the uridine H5/H6 atoms during a competition experiment in which Rev peptide was displaced from RRE-IIB by the higher affinity binding selected peptide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
De Guzman, R.N., Turner, R.B. & Summers, M.F. Biopolymers: Nucleic Acid Sciences 48, 181–195 (1998).
Patel, D.J. Curr. Opin. Struct. Biol. 9, 74–87 (1999).
Frankel, A.D. Curr. Opin. Struct. Biol. 10, 332–340 (2000).
Hermann, T. & Patel, D.J. Science 287, 820–825 (2000).
Tan, R., Chen, L., Buettner, J.A., Hudson, D. & Frankel, A.D. Cell 73, 1031–1040 (1993).
Battiste, J.L., et al. Science 273, 1547–1551 (1996).
Ye, X., Gorin, A., Ellington, A.D. & Patel, D.J. Nature Struct. Biol. 3, 1026–1033 (1996).
Ye, X. et al. Chem. Biol. 6, 657–669 (1999).
Harada, K., Martin, S.S. & Frankel, A.D. Nature 380, 175–179 (1996).
Harada, K., Martin, S.S., Tan, R. & Frankel, A.D. Proc. Natl. Acad. Sci. USA 94, 11887–11892 (1997).
Battiste, J.L., Tan, R., Frankel, A.D. & Williamson, J.R. Biochemistry 33, 2741–2747 (1994).
Peterson, R.D. & Feigon, J. J. Mol. Biol. 264, 863–877 (1996).
Hung, L.-W., Holbrook, E.L. & Holbrook, S.R. Proc. Natl. Acad. Sci. USA 97, 5107–5112 (2000).
Spera, S. & Bax, A. J. Am. Chem. Soc . 117, 5491–5495 (1991).
Kuboniwa, H., Grezesiek, S., Delaglio, F. & Bax, A. J. Biomol. NMR 4, 871–878 (1994).
Farrow, N.A. et al. Biochemistry 33, 5984–6003 (1994).
Dingley, A.J. & Grezesiek, S. J. Am. Chem. Soc. 120, 8293–8297 (1998).
Molinaro, M. & Tinoco, I. Nucleic Acids Res. 23, 3056–3063 (1995).
Calnan, B.J., Tidor, B., Biancalana, S., Hudson, D. & Frankel, A.D. Science 252, 1167–1171 (1991).
Hermann, T. & Westhof, E. Chem. Biol. 6, R335–R343 (1999).
Hermann, T. Angew. Chem. Int. Ed. 39, 1890–1905 (2000).
Le., S.-Y., Malim, M.H., Cullen, B.R. & Maizel, J.V. Nucleic Acids. Res. 18, 1613–1623 (1990).
Iwai, S., Pritchard, C., Mann, D.M., Karn, J. & Gait, M.J. Nucleic Acids Res. 20, 6465–6472 (1992).
Pritchard, C.E. et al. Nucleic Acids Res. 22, 2592–2600 (1994).
Cai, Z. et al. Nature Struct. Biol. 5, 203–212 (1998).
Delaglio, F. et al. J. Biomol. NMR 6, 277–293 (1995).
Johnson, B.A. & Blevins, R.A. J. Biomol. NMR 4, 603–614 (1994).
Nikonowicz, E.P. & Pardi, A. J. Mol. Biol . 232, 1141–1156 (1993).
Hu, W. et al. J. Biomol. NMR 12, 559–564 (1998)
Fiala, R., Jiang, F. & Sklenar, V. J. Biomol. NMR 12, 373–383 (1998).
Muhandiram, D.R. & Kay, L.E. J. Magn. Reson. B 103, 203–216 (1994).
Zwahlen, C. et al. J. Am. Chem. Soc. 119, 6711–6721 (1997).
Pearlman, D.A. et al. AMBER 4.1 (San Francisco, California; 1994).
Acknowledgements
This research was supported by a NIH grant to D.J.P. X.Ye was involved in the early stages of this project and S. Park provided technical assistance in the preparation of the labeled peptide.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gosser, Y., Hermann, T., Majumdar, A. et al. Peptide-triggered conformational switch in HIV-1 RRE RNA complexes. Nat Struct Mol Biol 8, 146–150 (2001). https://doi.org/10.1038/84138
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/84138
This article is cited by
-
Native mass spectrometry reveals the initial binding events of HIV-1 rev to RRE stem II RNA
Nature Communications (2020)
-
Potent inhibition of HIV-1 replication by backbone cyclic peptides bearing the Rev arginine rich motif
Journal of Biomedical Science (2007)
-
Protein-dependent ribozymes report molecular interactions in real time
Nature Biotechnology (2002)